Association of early HIV viremia with mortality after HIV-associated lymphoma
- PMID: 23736149
- PMCID: PMC3773290
- DOI: 10.1097/QAD.0b013e3283635232
Association of early HIV viremia with mortality after HIV-associated lymphoma
Abstract
Objective: To examine the association between early HIV viremia and mortality after HIV-associated lymphoma.
Design: Multicenter observational cohort study.
Setting: Center for AIDS Research Network of Integrated Clinical Systems cohort.
Participants: HIV-infected patients with lymphoma diagnosed between 1996 and 2011, who were alive 6 months after lymphoma diagnosis and with at least two HIV RNA values during the 6 months after lymphoma diagnosis.
Exposure: Cumulative HIV viremia during the 6 months after lymphoma diagnosis, expressed as viremia copy-6-months.
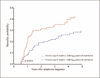
Main outcome measure: All-cause mortality between 6 months and 5 years after lymphoma diagnosis.
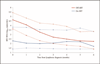
Results: Of 224 included patients, 183 (82%) had non-Hodgkin lymphoma (NHL) and 41 (18%) had Hodgkin lymphoma. At lymphoma diagnosis, 105 (47%) patients were on antiretroviral therapy (ART), median CD4⁺ cell count was 148 cells/μl (interquartile range 54-322), and 33% had suppressed HIV RNA (<400 copies/ml). In adjusted analyses, mortality was associated with older age [adjusted hazard ratio (AHR) 1.37 per decade increase, 95% CI 1.03-1.83], lymphoma occurrence on ART (AHR 1.63, 95% CI 1.02-2.63), lower CD4⁺ cell count (AHR 0.75 per 100 cells/μl increase, 95% CI 0.64-0.89), and higher early cumulative viremia (AHR 1.35 per log₁₀ copies × 6-months/ml, 95% CI 1.11-1.65). The detrimental effect of early cumulative viremia was consistent across patient groups defined by ART status, CD4⁺ cell count, and histology.
Conclusion: Exposure to each additional 1-unit log₁₀ in HIV RNA throughout the 6 months after lymphoma diagnosis was associated with a 35% increase in subsequent mortality. These results suggest that early and effective ART during chemotherapy may improve survival.
© 2013 Wolters Kluwer Health | Lippincott Williams & Wilkins
Conflict of interest statement
Conflicts of interest and source of funding: No conflict of interest declared; National Institutes of Health, UNC Lineberger Comprehensive Cancer Center
Figures

References
-
- Bonnet F, Burty C, Lewden C, Costagliola D, May T, Bouteloup V, et al. Changes in cancer mortality among HIV-infected patients: the Mortalite 2005 Survey. Clin Infect Dis. 2009;48:633–639. - PubMed
-
- Kaplan LD, Lee JY, Ambinder RF, Sparano JA, Cesarman E, Chadburn A, et al. Rituximab does not improve clinical outcome in a randomized phase 3 trial of CHOP with or without rituximab in patients with HIV-associated non-Hodgkin lym-phoma: AIDS-Malignancies Consortium Trial 010. Blood. 2005;106:1538–1543. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P30 AI027767/AI/NIAID NIH HHS/United States
- K01 TW009488/TW/FIC NIH HHS/United States
- R25 TW009340/TW/FIC NIH HHS/United States
- P30 AI36219/AI/NIAID NIH HHS/United States
- P30 AI50410/AI/NIAID NIH HHS/United States
- R24 AI067039/AI/NIAID NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
- 1R25TW009340-01/TW/FIC NIH HHS/United States
- 1K01TW009488-01/TW/FIC NIH HHS/United States
- T32 AI007140/AI/NIAID NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
- R21 CA180815/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

