The DcpS inhibitor RG3039 improves survival, function and motor unit pathologies in two SMA mouse models
- PMID: 23736298
- PMCID: PMC3781638
- DOI: 10.1093/hmg/ddt258
The DcpS inhibitor RG3039 improves survival, function and motor unit pathologies in two SMA mouse models
Abstract
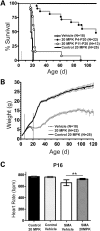
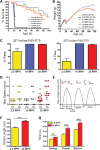
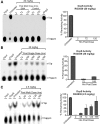
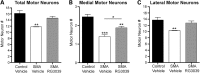
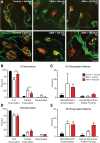
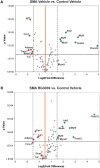
Spinal muscular atrophy (SMA) is caused by insufficient levels of the survival motor neuron (SMN) protein due to the functional loss of the SMN1 gene and the inability of its paralog, SMN2, to fully compensate due to reduced exon 7 splicing efficiency. Since SMA patients have at least one copy of SMN2, drug discovery campaigns have sought to identify SMN2 inducers. C5-substituted quinazolines increase SMN2 promoter activity in cell-based assays and a derivative, RG3039, has progressed to clinical testing. It is orally bioavailable, brain-penetrant and has been shown to be an inhibitor of the mRNA decapping enzyme, DcpS. Our pharmacological characterization of RG3039, reported here, demonstrates that RG3039 can extend survival and improve function in two SMA mouse models of varying disease severity (Taiwanese 5058 Hemi and 2B/- SMA mice), and positively impacts neuromuscular pathologies. In 2B/- SMA mice, RG3039 provided a >600% survival benefit (median 18 days to >112 days) when dosing began at P4, highlighting the importance of early intervention. We determined the minimum effective dose and the associated pharmacokinetic (PK) and exposure relationship of RG3039 and DcpS inhibition ex vivo. These data support the long PK half-life with extended pharmacodynamic outcome of RG3039 in 2B/- SMA mice. In motor neurons, RG3039 significantly increased both the average number of cells with gems and average number of gems per cell, which is used as an indirect measure of SMN levels. These studies contribute to dose selection and exposure estimates for the first studies with RG3039 in human subjects.
Figures



References
-
- Crawford T.O., Pardo C.A. The neurobiology of childhood spinal muscular atrophy. Neurobiol. Dis. 1996;3:97–110. - PubMed
-
- Munsat T.L., Davies K.E. International SMA consortium meeting (26–28 June 1992, Bonn, Germany) Neuromuscul. Disord. 1992;2:423–428. - PubMed
-
- Zerres K., Rudnik-Schoneborn S. Natural history in proximal spinal muscular atrophy. Clinical analysis of 445 patients and suggestions for a modification of existing classifications. Arch. Neurol. 1995;52:518–523. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

