Tyrosine supplementation for phenylketonuria
- PMID: 23737086
- PMCID: PMC7057270
- DOI: 10.1002/14651858.CD001507.pub3
Tyrosine supplementation for phenylketonuria
Update in
-
Tyrosine supplementation for phenylketonuria.Cochrane Database Syst Rev. 2021 Jan 4;1(1):CD001507. doi: 10.1002/14651858.CD001507.pub4. Cochrane Database Syst Rev. 2021. PMID: 33427303 Free PMC article.
Abstract
Background: Phenylketonuria is an inherited disease for which the main treatment is the dietary restriction of the amino acid phenylalanine. The diet has to be initiated in the neonatal period to prevent or reduce mental handicap. However, the diet is very restrictive and unpalatable and can be difficult to follow. A deficiency of the amino acid tyrosine has been suggested as a cause of some of the neuropsychological problems exhibited in phenylketonuria. Therefore, this review aims to assess the efficacy of tyrosine supplementation for phenylketonuria.
Objectives: To assess the effects of tyrosine supplementation alongside or instead of a phenylalanine-restricted diet for people with phenylketonuria, who commenced on diet at diagnosis and either continued on the diet or relaxed the diet later in life. To assess the evidence that tyrosine supplementation alongside, or instead of a phenylalanine-restricted diet improves intelligence, neuropsychological performance, growth and nutritional status, mortality rate and quality of life.
Search methods: We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group's Trials Register which is comprised of references identified from comprehensive electronic database searches, handsearches of relevant journals and abstract books of conference proceedings. Additional studies were identified from handsearches of the Journal of Inherited Metabolic Disease (from inception in 1978 to 1998). The manufacturers of prescribable dietary products used in the treatment of phenylketonuria were also contacted for further references.Date of the most recent search of the Group's Inborn Errors of Metabolism Trials Register: 28 June 2012.
Selection criteria: All randomised or quasi-randomised trials investigating the use of tyrosine supplementation versus placebo in people with phenylketonuria in addition to, or instead of, a phenylalanine-restricted diet. People treated for maternal phenylketonuria were excluded.
Data collection and analysis: Two authors independently assessed the trial eligibility, methodological quality and extracted the data.
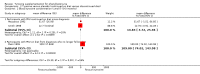
Main results: Six trials were found, of which three trials reporting the results of a total of 56 participants, were suitable for inclusion in the review. The blood tyrosine concentrations were significantly higher in the participants receiving tyrosine supplements than those in the placebo group, mean difference 23.46 (95% confidence interval 12.87 to 34.05). No significant differences were found between any of the other outcomes measured.
Authors' conclusions: From the available evidence no recommendations can be made about whether tyrosine supplementation should be introduced into routine clinical practice. Further randomised controlled studies are required to provide more evidence.
Conflict of interest statement
Current authors: Diana Webster has received travel expenses to attend conferences from the manufacturers of dietary products used in the treatment of PKU.
Previous authors: Vanessa Poustie and Tricia Rutherford have previously received travel expenses to attend conferences from the manufacturers of dietary products used in the treatment of PKU. Mrs Tricia Rutherford has been employed by Vitaflo, a manufacturer of protein substitutes, since September 2005; however, the protocol and the original review were completed prior to her commencing employment at this company.
Figures




Update of
-
Tyrosine supplementation for phenylketonuria.Cochrane Database Syst Rev. 2010 Aug 4;(8):CD001507. doi: 10.1002/14651858.CD001507.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Jun 05;(6):CD001507. doi: 10.1002/14651858.CD001507.pub3. PMID: 20687067 Updated.
References
References to studies included in this review
Mazzocco 1992 {published data only}
-
- Mazzocco MM, Yannicelli S, Nord AM, Doorninck W, Davidson‐Mundt AJ, Greene CL. Cognition and tyrosine supplementation among school‐aged children with phenylketonuria. American Journal of Diseases of Children 1992;146(11):1261‐4. - PubMed
Pietz 1995 {published data only}
-
- Pietz J, Landwehr R, Kutscha A, Schmidt H, Sonneville L, Trefz FK. Effect of high‐dose tyrosine supplementation on brain function in adults with phenylketonuria. Journal of Pediatrics 1995;127(6):936‐43. - PubMed
References to studies excluded from this review
Kalkanoglu 2005 {published data only}
-
- Kalkanoglu HS, Ahring KK, Sertkaya D, Moller LB, Romstad A, Mikkelsen I, et al. Behavioural effects of phenylalanine‐free amino acid tablet supplementation in intellectually disabled adults with untreated phenylketonuria. Acta Paediatrica 2005;94(9):1218‐22. - PubMed
Lou 1987 {published data only}
-
- Lou HC, Lykkelund C, Gerdes AM, Udesen H, Bruhn P. Increased vigilance and dopomine synthesis by large doses of tyrosine or phenylalanine restriction in phenylketonuria. Acta Paediatrica Scandanavia 1987;76(4):560‐5. - PubMed
Lykkelund 1988 {published data only}
-
- Lykkelund C, Nielsen JB, Lou HC, Rasmussen V, Gerdes AM, Christensen E, et al. Increased neurotransmitter biosynthesis in phenylketonuria induced by phenylalanine restriction or by supplementation of unrestricted diet with large amounts of tyrosine. European Journal of Pediatrics 1988;148(3):238‐45. - PubMed
MacDonald 2003 {published data only}
Wasser 1992 {published data only}
-
- Wasser S, Ettrich KU, Schmidt KD, Selle D, Theile H. Case studies of the effect of tyrosine administration in children with phenylketonuria on cognitive processes [Fallstudien zum einfluss von tyrosingaben bei phenylketonurischen kindern auf kognitive prozesse]. Klinische Padiatrie 1992;204(6):417‐21. - PubMed
References to studies awaiting assessment
Lines 1997 {published data only}
-
- Lines D, Magarey A, Raymond J, Robertson E. Tyrosine supplementation in phenylketonuria [letter]. Journal of Paediatrics and Child Health 1997;33(2):177. - PubMed
Additional references
Dixon 1994
-
- Dixon M. Disorders of amino acid metabolism, organic acidaemias and uria cycle defects. In: Shaw V, Lawson M editor(s). Clinical Paediatric Dietetics. Oxford: Blackwell Science, 1994:177‐209.
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analysis involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Lou 1985
-
- Lou HC, Guttler F, Lykkelund C, Bruhn P, Niederwieser A. Decreased vigilance and neurotransmitter synthesis after discontinuation of dietary treatment for phenylketonuria in adolescents. European Journal of Pediatrics 1985;144(1):17‐20. - PubMed
MRC1 1993
MRC2 1993
Oldendorf 1973
-
- Oldendorf WH. Saturation of blood brain barrier transport of amino acids in phenylketonuria. Archives of Neurology 1973;28(1):45‐8. - PubMed
Paine 1957
-
- Paine RS. The variability and manifestations of untreated patients with phenylketonuria (phenylpyruvic aciduria). Pediatrics 1957;20:290‐302. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273(5):408‐12. - PubMed
Scriver 1995
-
- Scriver CR, Kaufman S, Woo SLC. The hyperphenylalaninaemias. In: Scriver CR, Beaudet AL, Sly WS, Valle D editor(s). The Metabolic and Molecular Bases of Inherited Disease. 7th Edition. New York: McGraw‐Hill, 1995:1015‐76.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

