Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis
- PMID: 23737089
- PMCID: PMC6481746
- DOI: 10.1002/14651858.CD008037.pub3
Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis
Update in
-
Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis.Cochrane Database Syst Rev. 2020 Jul 16;7(7):CD008037. doi: 10.1002/14651858.CD008037.pub4. Cochrane Database Syst Rev. 2020. PMID: 32671834 Free PMC article.
Abstract
Background: Cystic fibrosis is a multi-system disease characterised by the production of thick secretions causing recurrent pulmonary infection, often with unusual bacteria. This leads to lung destruction and eventually death through respiratory failure. There are no antibiotics in development that exert a new mode of action and many of the current antibiotics are ineffective in eradicating the bacteria once chronic infection is established. Antibiotic adjuvants - therapies that act by rendering the organism more susceptible to attack by antibiotics or the host immune system, by rendering it less virulent or killing it by other means, are urgently needed.
Objectives: To determine if antibiotic adjuvants improve clinical and microbiological outcome of pulmonary infection in people with cystic fibrosis.
Search methods: We searched the Cystic Fibrosis Trials Register which is compiled from database searches, hand searches of appropriate journals and conference proceedings.Date of most recent search: 26 July 2012.We also searched MEDLINE (all years) on 23 February 2013 and ongoing trials registers on 13 February 2013.
Selection criteria: Randomised controlled trials and quasi-randomised controlled trials of a therapy exerting an antibiotic adjuvant mechanism of action compared to placebo or no therapy for people with cystic fibrosis.
Data collection and analysis: The authors independently assessed and extracted data from identified studies.
Main results: We identified eighteen studies of which four are included that examined antibiotic adjuvant therapies, three studies are ongoing. The included studies involve the assessment of β-carotene, garlic and zinc supplementation and KB001 (a biological agent). No therapy demonstrated a significant effect upon pulmonary function, pulmonary exacerbations or quality of life. The study of zinc supplementation reports a reduction in the requirement of oral antibiotics but not of intravenous antibiotics, an effect that is difficult to understand.
Authors' conclusions: We could not identify an antibiotic adjuvant therapy that could be recommended for the treatment of lung infection in those with cystic fibrosis. The emergence of increasingly resistant bacteria makes the reliance on antibiotics alone challenging for cystic fibrosis teams. There is a need to explore alternative strategies, such as the use of adjuvant therapies. Further research is required to provide future therapeutic options.
Conflict of interest statement
He has also acted as a consultant to the pharmaceutical industry for therapies in development for pulmonary infection with
Novartis ‐ dry powder inhaled tobramycin
Mpex Pharmaceuticals ‐ inhaled, nebulised levofloxacin
Biocontrol ‐ bacteriophage therapy
Professor Smyth has also received payment for commercial clinical trials:
Boehringer Ingelheim, Ltd. (UK) ‐ inhaled tiotropium as a long‐acting bronchodilator in CF.
Figures




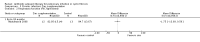
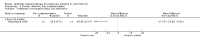
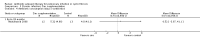
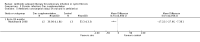











Update of
-
Antibiotic adjuvant therapy for pulmonary infection in cystic fibrosis.Cochrane Database Syst Rev. 2010 Oct 6;(10):CD008037. doi: 10.1002/14651858.CD008037.pub2. Cochrane Database Syst Rev. 2010. Update in: Cochrane Database Syst Rev. 2013 Jun 05;(6):CD008037. doi: 10.1002/14651858.CD008037.pub3. PMID: 20927769 Updated.
References
References to studies included in this review
Abdulhamid 2008 {published and unpublished data}
Milla 2013 {unpublished data only}
-
- Milla C, Molfino NA for KaloBios Pharmaceuticals. A phase I/II randomized, double‐blind, placebo‐controlled, single‐dose, dose escalation study of KB001 in cystic fibrosis patients infected with Pseudomonas aeruginosa. www.clinicaltrials.gov/ct2/show/study/NCT00638365?term=NCT00638365&r... (accessed 21 March 2013).
Renner 2001 {published data only}
-
- Engl B, Rust P, Eichler I, Renner S, Elmadfa I. Bioavailability of therapeutic Beta‐Carotine (BC) in patients with cystic fibrosis (CF) and effects on anthropometrical parameters over 6 months [abstract]. Monatsschrift fur Kinderheilkunde 1997;145:S 134.
-
- Renner S, Wojnarowski C, Koller DY, Rust P, Elmadfa I, Eichler I. Patients with cystic fibrosis (CF) benefit from ß‐Carotene supplementation for 6 months [abstract]. Pediatric Pulmonology 1997;24(Suppl 15):314.
-
- Renner S, Wojnarowski C, Koller DY, Rust P, Elmadfa I, Eichler I. Patients with cystic fibrosis (CF) benefit from ß‐Carotene supplementation for 6 months [abstract]. Proceedings of: 22nd European Cystic Fibrosis Conference; 1998 June 13‐19; Berlin, Germany. 1998:97.
-
- Rust P, Eichler I, Elmadfa I. Influence of an oral beta‐carotene supplementation on the antioxidant status of patients with cystic fibrosis [abstract]. Atemwegs und Lungenkrankheiten 1997;23:51.
Smyth 2010 {published data only}
-
- Smyth A, Cifelli P, Lewis S, Erskine P, Holland E, Willaims P, et al. Quorum sensing molecules in sputum and plasma as biomarkers in patients with CF and chronic pseudomonas aeruginosa [abstract]. Pediatric Pulmonology 2008;43(Suppl 31):337.
-
- Smyth A, Cifelli P, Lewis S, Erskine P, Holland E, Williams P, et al. A randomized controlled trial of macerated garlic oil in patients with CF and chronic pseudomonas aeruginosa [abstract]. Pediatric Pulmonology 2008;43(Suppl 31):336.
-
- Smyth AR, Cifelli PM, Ortori CA, Righetti K, Lewis S, Erskine P, et al. Garlic as an inhibitor of Pseudomonas aeruginosa quorum sensing in cystic fibrosis – a pilot randomised controlled trial. Pediatric Pulmonology 2010;45(4):356‐62. - PubMed
References to studies excluded from this review
Brown 1985 {published data only}
-
- Brown J, Mellis CM, Wood RE. EDTA aerosol in pseudomonal lung infection [abstract]. Proceedings of 9th International Cystic Fibrosis Congress; 1984 June 9‐15; Brighton, England. 1984:4.10.
-
- Brown J, Mellis CM, Wood RE. Edetate sodium aerosol in Pseudomonas lung infection in cystic fibrosis. American Journal of Diseases of Children 1985;139(8):836‐9. - PubMed
Durairaj 2007 {published data only}
-
- Durairaj L, Launspach J, Watt JL, Mohamad Z, Kline J, Zabner J. Safety assessment of inhaled xylitol in subjects with cystic fibrosis. Journal of Cystic Fibrosis 2007 Jan;6(1):31‐4. - PubMed
Gontijo‐Amaral 2012 {published data only}
-
- Gontijo‐Amaral C, Guimaraes EV, Camargos P. Oral magnesium supplementation in children with cystic fibrosis improves clinical and functional variables: a double‐blind,randomized, placebo‐controlled crossover trial. American Journal of Clinical Nutrition 2012;96(1):50‐6. [DOI: 10.3945/ajcn.112.034207] - DOI - PubMed
Grasemann 2013 {published data only}
Hauber 2008 {published data only}
Homnick 1995 {published data only}
-
- Homnick DN, Spillers CR, Cox SR, Cox JH, Yelton LA, DeLoof MJ, et al. Single‐ and multiple‐dose‐response relationships of beta‐carotene in cystic fibrosis.[see comment]. Journal of Pediatrics 1995 Sep;127(3):491‐4. - PubMed
Kollberg 2003 {published data only}
-
- Kollberg H, Carlander D, Olesen H, Wejaker PE, Johannesson M, Larsson A. Oral administration of specific yolk antibodies (IgY) may prevent Pseudomonas aeruginosa infections in patients with cystic fibrosis: a phase I feasibility study. Pediatric Pulmonology 2003 Jun;35(6):433‐40. - PubMed
-
- Nilsson E, Kollberg H, Johannesson M, Wejaker PE, Carlander D, Larsson A. More than 10 years' continuous oral treatment with specific immunoglobulin Y for the prevention of Pseudomonas aeruginosa infections: a case report. Journal of Medicinal Food 2007;10(2):375‐8. - PubMed
-
- Nilsson E, Larsson A, Olesen HV, Wejaker PE, Kollberg H. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatric Pulmonology 2008;43(9):892‐9. - PubMed
Kollberg 2010 {published data only}
-
- Kollberg H, Larsson A, Nilsson E. Anti‐pseudomonas IGY ready for phase III [abstract]. Pediatric Pulmonology 2010;45(Suppl 33):343, Abstract no: 345. [CFGD Register: PI252a]
-
- Larsson A. Phase III study (IMPACTT) on anti‐pseudomonas IgY [abstract]. Journal of Cystic Fibrosis 2011;10(Suppl 1):S24, Abstract no: 92. [CFGD Register: PI252b]
Kutateladze 2008 {published data only}
-
- Kutateladze M, Adamia R. Phage therapy experience at the Eliava Institute. Medecine Et Maladies Infectieuses 2008;38(8):426‐30. - PubMed
Lands 2010 {published data only}
Lebecque 2008 {unpublished data only}
-
- Lebecque P. Single center, double‐blind, randomized, placebo‐controlled, two‐period/two‐treatment crossover study investigating the effect of miglustat on the nasal potential difference in patients with cystic fibrosis homozygous for the F508del mutation. clinicaltrials.gov/ct2/show/NCT00742092?term=NCT00742092&rank=1 (accessed 21 March 2013).
Leonard 2012 {published data only}
Moss 2012 {published data only}
Olveira 2010 {published data only}
-
- Olveira G, Olveira C, Acosta E, Espíldora F, Garrido‐Sánchez L, García‐Escobar E. Fatty acid supplements improve respiratory, inflammatory and nutritional parameters in adults with cystic fibrosis. Archivos de Bronconeumologia 2010;46(2):70‐7. - PubMed
Panchaud 2006 {published data only}
-
- Panchaud A, Sauty A, Kernen Y, Decosterd LA, Buclin T, Boulat O, et al. Biological effects of a dietary omega‐3 polyunsaturated fatty acids supplementation in cystic fibrosis patients: a randomized, crossover placebo‐controlled trial. Clinical Nutrition 2006; Vol. 25, issue 3:418‐27. [DOI: 10.1016/j.clnu.2005.10.011] - DOI - PubMed
Safai‐Kutti 1991 {published data only}
-
- Safai‐Kutti S, Selin E, Larsson S, Jagenburg R, Sten G, Kjellmer I. Zinc therapy in children with cystic fibrosis. Beitraege zur Infusionstherapie 1991;27:104‐14. - PubMed
Sagel 2011 {published data only}
Schuster 2011 {published data only}
-
- Schuster A. Phase III study to evaluate clinical efficacy and safety of avian polyclonal anti‐Pseudomonas antibodies (IgY) in prevention of recurrence of Pseudomonas aeruginosa infection in cystic fibrosis patients. http://clinicaltrials.gov/ct2/show/NCT01455675?term=NCT01455675&rank=1 (accessed 21 March 2013).
Winklhofer‐Roob 1995 {published data only}
-
- Winklhofer‐Roob BM, Puhl H, Khoschsorur G, van't Hof MA, Esterbauer H, Shmerling DH. Enhanced resistance to oxidation of low density lipoproteins and decreased lipid peroxide formation during beta‐carotene supplementation in cystic fibrosis. Free Radical Biology & Medicine 1995;18(5):849‐59. - PubMed
-
- Winklhofer‐Roob BM, Schlegel‐Haueter SE, Khoschsorur G, van't Hof MA, Suter S, Shmerling DH. Neutrophil elastase/alpha 1‐proteinase inhibitor complex levels decrease in plasma of cystic fibrosis patients during long‐term oral beta‐carotene supplementation. Pediatric Research 1996;40(1):130‐4. - PubMed
-
- Winklhofer‐Roob BM, van't Hof MA, Shmerling DH. Response to oral beta‐carotene supplementation in patients with cystic fibrosis: a 16‐month follow‐up study.[erratum appears in Acta Paediatr 1996 Jan;85(1):124]. Acta Paediatrica 1995 Oct;84(10):1132‐6. - PubMed
References to ongoing studies
Molfino 2012 {unpublished data only}
-
- Study to evaluate the effect of KB001‐A on time‐to‐need for antibiotic treatment.. Ongoing study December 2012..
Walshaw 2011 {unpublished data only}
-
- A double‐blind, randomized, placebo‐controlled, cross‐over study to evaluate the safety, tolerability and preliminary efficacy of alginate oligosaccharide (OligoG) administered for 28 days in subjects with cystic fibrosis chronically colonised with Pseudomonas aeruginosa.. Ongoing study May 2011..
Zabner 2009 {unpublished data only}
-
- Aerosolized hypertonic xylitol versus hypertonic saline in cystic fibrosis (CF) subjects.. Ongoing study June 2009..
Additional references
Borysowski 2006
-
- Borysowski J, Weber‐Dbrowska B, Górski A. Bacteriophage endolysins as a novel class of antibacterial agents. Experimental Biology and Medicine 2006;231(4):366‐77. [PUBMED: 16565432] - PubMed
Dodge 2007
Druesne‐Pecollo 2010
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johansson 2008
-
- Johansson EM, Crusz SA, Kolomiets E, Buts L, Kadam RU, Cacciarini M, et al. Inhibition and dispersion of Pseudomonas aeruginosa biofilms by glycopeptide dendrimers targeting the fucose‐specific lectin LecB. Chemistry and Biology 2008;15(12):1249‐57. [DOI: 10.1016/j.chembiol.2008.10.009] - DOI - PubMed
Langton‐Hewer 2009
Lee 2004
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rosenfeld 2001
Rosenstein 1998
UK Cystic Fibrosis Trust 2004
-
- UK Cystic Fibrosis Trust Infection Control Group. Pseudomonas aeruginosa infection in people with cystic fibrosis: Suggestions for prevention and infection control. Report of the UK Cystic Fibrosis Infection Control Group 2004. [ISBN: 0‐9540536‐9‐9]
UK Cystic Fibrosis Trust Antibiotic Working Group
-
- UK Cystic FIbrosis Antibiotic Working Group. Antibiotic Treatment for Cystic Fibrosis ‐ 3rd Edition. Cystic Fibrosis Trust 2009.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

