The adventitia: Essential role in pulmonary vascular remodeling
- PMID: 23737168
- PMCID: PMC4169049
- DOI: 10.1002/cphy.c090017
The adventitia: Essential role in pulmonary vascular remodeling
Abstract
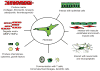
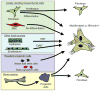
A rapidly emerging concept is that the vascular adventitia acts as a biological processing center for the retrieval, integration, storage, and release of key regulators of vessel wall function. It is the most complex compartment of the vessel wall and comprises a variety of cells including fibroblasts, immunomodulatory cells, resident progenitor cells, vasa vasorum endothelial cells, and adrenergic nerves. In response to vascular stress or injury, resident adventitial cells are often the first to be activated and reprogrammed to then influence tone and structure of the vessel wall. Experimental data indicate that the adventitial fibroblast, the most abundant cellular constituent of adventitia, is a critical regulator of vascular wall function. In response to vascular stresses such as overdistension, hypoxia, or infection, the adventitial fibroblast is activated and undergoes phenotypic changes that include proliferation, differentiation, and production of extracellular matrix proteins and adhesion molecules, release of reactive oxygen species, chemokines, cytokines, growth factors, and metalloproteinases that, collectively, affect medial smooth muscle cell tone and growth directly and that stimulate recruitment and retention of circulating inflammatory and progenitor cells to the vessel wall. Resident dendritic cells also participate in "sensing" vascular stress and actively communicate with fibroblasts and progenitor cells to simulate repair processes that involve expansion of the vasa vasorum, which acts as a conduit for further delivery of inflammatory/progenitor cells. This review presents the current evidence demonstrating that the adventitia acts as a key regulator of pulmonary vascular wall function and structure from the "outside in."
© 2011 American Physiological Society.
Figures



References
-
- Arciniegas E, Frid MG, Douglas IS, Stenmark KR. Perspectives on endothelial-to-mesenchymal transition: | Potential contribution to vascular remodeling in chronic pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1–L8. - PubMed
-
- Ardanaz N, Pagano PJ. Hydrogen peroxide as a paracrine vascular mediator: Regulation and signaling leading to dysfunction. Exp Biol Med (Maywood) 2006;231:237–251. - PubMed
-
- Arribas SM, Hillier C, Gonzalez C, McGrory S, Dominiczak AF, McGrath JC. Cellular aspects of vascular remodeling in hypertension revealed by confocal microscopy. Hypertension. 1997;30:1455–1464. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

