Physiology of endothelin and the kidney
- PMID: 23737206
- PMCID: PMC3940435
- DOI: 10.1002/cphy.c100039
Physiology of endothelin and the kidney
Abstract
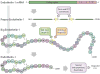
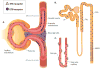
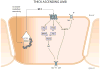
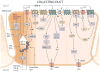
Since its discovery in 1988 as an endothelial cell-derived peptide that exerts the most potent vasoconstriction of any known endogenous compound, endothelin (ET) has emerged as an important regulator of renal physiology and pathophysiology. This review focuses on how the ET system impacts renal function in health; it is apparent that ET regulates multiple aspects of kidney function. These include modulation of glomerular filtration rate and renal blood flow, control of renin release, and regulation of transport of sodium, water, protons, and bicarbonate. These effects are exerted through ET interactions with almost every cell type in the kidney, including mesangial cells, podocytes, endothelium, vascular smooth muscle, every section of the nephron, and renal nerves. In addition, while not the subject of the current review, ET can also indirectly affect renal function through modulation of extrarenal systems, including the vasculature, nervous system, adrenal gland, circulating hormones, and the heart. As will become apparent, these pleiotropic effects of ET are of fundamental physiologic importance in the control of renal function in health. In addition, to help put these effects into perspective, we will also discuss, albeit to a relatively limited extent, how alterations in the ET system can contribute to hypertension and kidney disease.
© 2011 American Physiological Society. Compr Physiol 1:699-729, 2011.
Figures



References
-
- Abassi Z, Ellahham S, Winaver J, Hoffman A. The intrarenal endothelin system and hypertension. News Physiol Sci. 2001;16:152–156. - PubMed
-
- Abassi ZA, Tate JE, Golomb E, Keiser HR. Role of neutral endopeptidase in the metabolism of endothelin. Hypertension. 1992;20:89–95. - PubMed
-
- Ackermann M, Ritthaler T, Riegger G, Kurtz A, Kramer BK. Endothelin inhibits cAMP-induced renal release from isolated renal juxtaglomerular cells. Journal of Cardiovascular Pharmacology. 1995;26:S135–S137. - PubMed
-
- Akabane S, Yoshimi H, Yoshida K, Kawano Y, Ashida T, Kinoshita O, Matsushima Y, Kawamura M, Imanishi M, Kuramochi M, Omae T. Differential effects of endothelin-1 and big endothelin on canine kidneys. J Cardiovasc Pharmacol. 1991;17(Suppl. 7):S302–S304. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

