Angiogenesis and intramembranous osteogenesis
- PMID: 23737393
- PMCID: PMC3803110
- DOI: 10.1002/dvdy.23992
Angiogenesis and intramembranous osteogenesis
Abstract
Background: Angiogenesis is likely critical for the process of intramembranous osteogenesis; however, the developmental relationship between blood vessels and bone mineralization is not well studied within intramembranous bones. Given its importance, changes in angiogenesis regulation are likely to contribute to evolutionarily and medically relevant craniofacial variation.
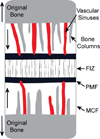
Results: We summarize what is known about the association between angiogenesis and intramembranous osteogenesis, supplementing with information from the better-studied processes of endochondral ossification and distraction osteogenesis. Based on this review, we introduce a model of angiogenesis during early intramembranous osteogenesis as well as a series of null hypotheses to be tested.
Conclusions: This model can serve as a basis of future research on the spatio-temporal association and regulatory interactions of mesenchymal, vascular, and bone cells, which will be required to illuminate the potential effects of angiogenesis dysregulation on craniofacial skeletal phenotypes.
Keywords: craniofacial development; intramembranous ossification; vascular invasion.
Copyright © 2013 Wiley Periodicals, Inc.
Figures




References
-
- Abzhanov A, Rodda SJ, McMahon AP, Tabin CJ. Regulation of skeletogenic differentiation in cranial dermal bone. Development. 2007;134:3133–3144. - PubMed
-
- Amizuka N, Hasegawa T, Oda K, Luiz de Freitas P, Hoshi K, Li M, Ozawa H. Histology of epiphyseal cartilage calcification and endochondral ossification. Front Biosci. 2012;4:2085–2100. - PubMed
-
- Aronson J, Good B, Stewart C, Harrison B, Harp J. Preliminary studies of mineralization during distraction osteogenesis. Clinical Orthopaedics and Related Research. 1990;250:43–49. - PubMed
-
- Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clinical Orthopaedics and Related Research. 1994;301:124–131. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

