Anti-antimicrobial peptides: folding-mediated host defense antagonists
- PMID: 23737519
- PMCID: PMC3711284
- DOI: 10.1074/jbc.M113.459560
Anti-antimicrobial peptides: folding-mediated host defense antagonists
Abstract
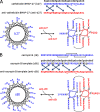
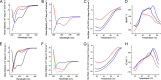
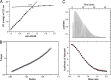
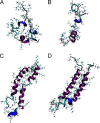
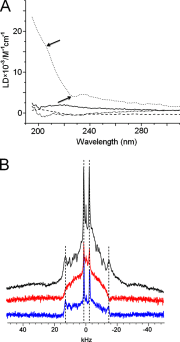
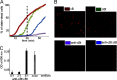
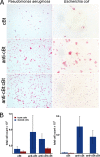
Antimicrobial or host defense peptides are innate immune regulators found in all multicellular organisms. Many of them fold into membrane-bound α-helices and function by causing cell wall disruption in microorganisms. Herein we probe the possibility and functional implications of antimicrobial antagonism mediated by complementary coiled-coil interactions between antimicrobial peptides and de novo designed antagonists: anti-antimicrobial peptides. Using sequences from native helical families such as cathelicidins, cecropins, and magainins we demonstrate that designed antagonists can co-fold with antimicrobial peptides into functionally inert helical oligomers. The properties and function of the resulting assemblies were studied in solution, membrane environments, and in bacterial culture by a combination of chiroptical and solid-state NMR spectroscopies, microscopy, bioassays, and molecular dynamics simulations. The findings offer a molecular rationale for anti-antimicrobial responses with potential implications for antimicrobial resistance.
Keywords: Antimicrobial Peptides; Membrane Biophysics; Microscopy; Molecular Dynamics; Nuclear Magnetic Resonance; Protein Design; Protein Folding.
Figures
Similar articles
-
Studying the Interaction of Magainin 2 and Cecropin A with E. coli Bacterial Cells Using Circular Dichroism.Methods Mol Biol. 2017;1548:247-253. doi: 10.1007/978-1-4939-6737-7_17. Methods Mol Biol. 2017. PMID: 28013509
-
Synthetic cationic amphiphilic α-helical peptides as antimicrobial agents.Biomaterials. 2011 Mar;32(8):2204-12. doi: 10.1016/j.biomaterials.2010.11.054. Epub 2010 Dec 18. Biomaterials. 2011. PMID: 21168911
-
Structural contributions to the intracellular targeting strategies of antimicrobial peptides.Biochim Biophys Acta. 2010 Oct;1798(10):1934-43. doi: 10.1016/j.bbamem.2010.07.003. Epub 2010 Jul 15. Biochim Biophys Acta. 2010. PMID: 20637722 Free PMC article.
-
Insights into the mechanisms of action of host defence peptides from biophysical and structural investigations.J Pept Sci. 2011 May;17(5):306-14. doi: 10.1002/psc.1343. Epub 2011 Feb 28. J Pept Sci. 2011. PMID: 21360627 Review.
-
[Cationic antimicrobial peptides: from innate immunity study to drug development. Up date].Med Mal Infect. 2007 Apr;37(4):194-9. doi: 10.1016/j.medmal.2006.09.009. Epub 2007 Feb 15. Med Mal Infect. 2007. PMID: 17306486 Review. French.
Cited by
-
Activated Polyhydroxyalkanoate Meshes Prevent Bacterial Adhesion and Biofilm Development in Regenerative Medicine Applications.Front Bioeng Biotechnol. 2020 May 15;8:442. doi: 10.3389/fbioe.2020.00442. eCollection 2020. Front Bioeng Biotechnol. 2020. PMID: 32671021 Free PMC article.
-
Engineering monolayer poration for rapid exfoliation of microbial membranes.Chem Sci. 2017 Feb 1;8(2):1105-1115. doi: 10.1039/c6sc02925f. Epub 2016 Sep 26. Chem Sci. 2017. PMID: 28451250 Free PMC article.
-
Anticancer activities of natural antimicrobial peptides from animals.Front Microbiol. 2024 Jan 17;14:1321386. doi: 10.3389/fmicb.2023.1321386. eCollection 2023. Front Microbiol. 2024. PMID: 38298540 Free PMC article. Review.
-
Autonomously folded α-helical lockers promote RNAi.Sci Rep. 2016 Oct 10;6:35012. doi: 10.1038/srep35012. Sci Rep. 2016. PMID: 27721465 Free PMC article.
-
Switching Cytolytic Nanopores into Antimicrobial Fractal Ruptures by a Single Side Chain Mutation.ACS Nano. 2021 Jun 22;15(6):9679-9689. doi: 10.1021/acsnano.1c00218. Epub 2021 Apr 22. ACS Nano. 2021. PMID: 33885289 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

