Transforming growth factor-β induces transcription factors MafK and Bach1 to suppress expression of the heme oxygenase-1 gene
- PMID: 23737527
- PMCID: PMC3711329
- DOI: 10.1074/jbc.M113.450478
Transforming growth factor-β induces transcription factors MafK and Bach1 to suppress expression of the heme oxygenase-1 gene
Abstract
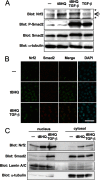
Transforming growth factor-β (TGF-β) has multiple functions in embryogenesis, adult homeostasis, tissue repair, and development of cancer. Here, we report that TGF-β suppresses the transcriptional activation of the heme oxygenase-1 (HO-1) gene, which is implicated in protection against oxidative injury and lung carcinogenesis. HO-1 is a target of the oxidative stress-responsive transcription factor Nrf2. TGF-β did not affect the stabilization or nuclear accumulation of Nrf2 after stimulation with electrophiles. Instead, TGF-β induced expression of transcription factors MafK and Bach1. Enhanced expression of either MafK or Bach1 was enough to suppress the electrophile-inducible expression of HO-1 even in the presence of accumulated Nrf2 in the nucleus. Knockdown of MafK and Bach1 by siRNA abolished TGF-β-dependent suppression of HO-1. Furthermore, chromatin immunoprecipitation assays revealed that Nrf2 substitutes for Bach1 at the antioxidant response elements (E1 and E2), which are responsible for the induction of HO-1 in response to oxidative stress. On the other hand, pretreatment with TGF-β suppressed binding of Nrf2 to both E1 and E2 but marginally increased the binding of MafK to E2 together with Smads. As TGF-β is activated after tissue injury and in the process of cancer development, these findings suggest a novel mechanism by which damaged tissue becomes vulnerable to oxidative stress and xenobiotics.
Keywords: Cancer; Cancer Prevention; Heme Oxygenase; Nrf2; Oxidative Stress; Transcriptional Regulation; Transforming Growth Factor β (TGFβ).
Figures






References
-
- Derynck R., Miyazono K. (eds) (2008) The TGF-β Family, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
-
- Massagué J., Seoane J., Wotton D. (2005) Smad transcription factors. Genes Dev. 19, 2783–2810 - PubMed
-
- Siegel P. M., Massagué J. (2003) Cytostatic and apoptotic actions of TGF-β in homeostasis and cancer. Nat. Rev. Cancer 3, 807–821 - PubMed
-
- Akhurst R. J., Derynck R. (2001) TGF-β signaling in cancer—a double-edged sword. Trends Cell Biol. 11, S44–S51 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

