Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine
- PMID: 23737747
- PMCID: PMC3667758
- DOI: 10.1371/journal.ppat.1003389
Antibody quality and protection from lethal Ebola virus challenge in nonhuman primates immunized with rabies virus based bivalent vaccine
Abstract
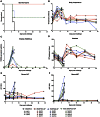
We have previously described the generation of a novel Ebola virus (EBOV) vaccine platform based on (a) replication-competent rabies virus (RABV), (b) replication-deficient RABV, or (c) chemically inactivated RABV expressing EBOV glycoprotein (GP). Mouse studies demonstrated safety, immunogenicity, and protective efficacy of these live or inactivated RABV/EBOV vaccines. Here, we evaluated these vaccines in nonhuman primates. Our results indicate that all three vaccines do induce potent immune responses against both RABV and EBOV, while the protection of immunized animals against EBOV was largely dependent on the quality of humoral immune response against EBOV GP. We also determined if the induced antibodies against EBOV GP differ in their target, affinity, or the isotype. Our results show that IgG1-biased humoral responses as well as high levels of GP-specific antibodies were beneficial for the control of EBOV infection after immunization. These results further support the concept that a successful EBOV vaccine needs to induce strong antibodies against EBOV. We also showed that a dual vaccine against RABV and filoviruses is achievable; therefore addressing concerns for the marketability of this urgently needed vaccine.
Conflict of interest statement
I have read the journal's policy and have the following conflict: pending patent application entitled “US Prov. Appl. MULTIVALENT VACCINES FOR RABIES VIRUS AND FILOVIRUS”. This does not alter our adherence to all PLOS Pathogens policies on sharing data and materials.
Figures




References
-
- Sanchez A, Geisbert TW, Feldmann H (2007) Filoviridae: Marburg and Ebola viruses. In: Knipe DM, Howley PM, Griffin DE, editors. Fields virology, 5th ed. Philadelphia, PA.: Lippincott Williams & Wilkins. pp. 1409–1448.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

