CDK4 T172 phosphorylation is central in a CDK7-dependent bidirectional CDK4/CDK2 interplay mediated by p21 phosphorylation at the restriction point
- PMID: 23737759
- PMCID: PMC3667761
- DOI: 10.1371/journal.pgen.1003546
CDK4 T172 phosphorylation is central in a CDK7-dependent bidirectional CDK4/CDK2 interplay mediated by p21 phosphorylation at the restriction point
Abstract
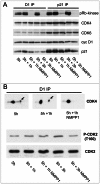
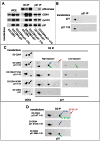
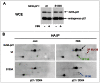
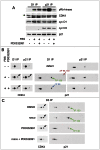
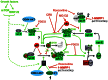
Cell cycle progression, including genome duplication, is orchestrated by cyclin-dependent kinases (CDKs). CDK activation depends on phosphorylation of their T-loop by a CDK-activating kinase (CAK). In animals, the only known CAK for CDK2 and CDK1 is cyclin H-CDK7, which is constitutively active. Therefore, the critical activation step is dephosphorylation of inhibitory sites by Cdc25 phosphatases rather than unrestricted T-loop phosphorylation. Homologous CDK4 and CDK6 bound to cyclins D are master integrators of mitogenic/oncogenic signaling cascades by initiating the inactivation of the central oncosuppressor pRb and cell cycle commitment at the restriction point. Unlike the situation in CDK1 and CDK2 cyclin complexes, and in contrast to the weak but constitutive T177 phosphorylation of CDK6, we have identified the T-loop phosphorylation at T172 as the highly regulated step determining CDK4 activity. Whether both CDK4 and CDK6 phosphorylations are catalyzed by CDK7 remains unclear. To answer this question, we took a chemical-genetics approach by using analogue-sensitive CDK7(as/as) mutant HCT116 cells, in which CDK7 can be specifically inhibited by bulky adenine analogs. Intriguingly, CDK7 inhibition prevented activating phosphorylations of CDK4/6, but for CDK4 this was at least partly dependent on its binding to p21 (cip1) . In response to CDK7 inhibition, p21-binding to CDK4 increased concomitantly with disappearance of the most abundant phosphorylation of p21, which we localized at S130 and found to be catalyzed by both CDK4 and CDK2. The S130A mutation of p21 prevented the activating CDK4 phosphorylation, and inhibition of CDK4/6 and CDK2 impaired phosphorylations of both p21 and p21-bound CDK4. Therefore, specific CDK7 inhibition revealed the following: a crucial but partly indirect CDK7 involvement in phosphorylation/activation of CDK4 and CDK6; existence of CDK4-activating kinase(s) other than CDK7; and novel CDK7-dependent positive feedbacks mediated by p21 phosphorylation by CDK4 and CDK2 to sustain CDK4 activation, pRb inactivation, and restriction point passage.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Sherr CJ (1995) D-type cyclins. Trends Biochem Sci 20: 187–190. - PubMed
-
- Bartek J, Bartkova J, Lukas J (1996) The retinoblastoma protein pathway and the restriction point. Curr Opin Cell Biol 8: 805–814. - PubMed
-
- Yao G, Lee TJ, Mori S, Nevins JR, You L (2008) A bistable Rb-E2F switch underlies the restriction point. Nat Cell Biol 10: 476–482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

