Predicting the Drug Safety for Traditional Chinese Medicine through a Comparative Analysis of Withdrawn Drugs Using Pharmacological Network
- PMID: 23737823
- PMCID: PMC3657406
- DOI: 10.1155/2013/256782
Predicting the Drug Safety for Traditional Chinese Medicine through a Comparative Analysis of Withdrawn Drugs Using Pharmacological Network
Abstract
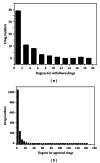
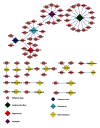
As the major issue to limit the use of drugs, drug safety leads to the attrition or failure in clinical trials of drugs. Therefore, it would be more efficient to minimize therapeutic risks if it could be predicted before large-scale clinical trials. Here, we integrated a network topology analysis with cheminformatics measurements on drug information from the DrugBank database to detect the discrepancies between approved drugs and withdrawn drugs and give drug safety indications. Thus, 47 approved drugs were unfolded with higher similarity measurements to withdrawn ones by the same target and confirmed to be already withdrawn or discontinued in certain countries or regions in subsequent investigations. Accordingly, with the 2D chemical fingerprint similarity calculation as a medium, the method was applied to predict pharmacovigilance for natural products from an in-house traditional Chinese medicine (TCM) database. Among them, Silibinin was highlighted for the high similarity to the withdrawn drug Plicamycin although it was regarded as a promising drug candidate with a lower toxicity in existing reports. In summary, the network approach integrated with cheminformatics could provide drug safety indications effectively, especially for compounds with unknown targets or mechanisms like natural products. It would be helpful for drug safety surveillance in all phases of drug development.
Figures



References
-
- Stevens JL, Baker TK. The future of drug safety testing: expanding the view and narrowing the focus. Drug Discovery Today. 2009;14(3-4):162–167. - PubMed
-
- Abernethy DR, Woodcock J, Lesko LJ. Pharmacological mechanism-based drug safety assessment and prediction. Clinical Pharmacology and Therapeutics. 2011;89(6):793–797. - PubMed
-
- Olson H, Betton G, Robinson D, et al. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regulatory Toxicology and Pharmacology. 2000;32(1):56–67. - PubMed
-
- Lu Z. Technical challenges in designing post-marketing eCRFs to address clinical safety and pharmacovigilance needs. Contemporary Clinical Trials. 2010;31(1):108–118. - PubMed
-
- Abraham J, Davis C. A comparative analysis of drug safety withdrawals in the UK and the US (1971–1992): implications for current regulatory thinking and policy. Social Science and Medicine. 2005;61(5):881–892. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

