Central precocious puberty caused by mutations in the imprinted gene MKRN3
- PMID: 23738509
- PMCID: PMC3808195
- DOI: 10.1056/NEJMoa1302160
Central precocious puberty caused by mutations in the imprinted gene MKRN3
Abstract
Background: The onset of puberty is first detected as an increase in pulsatile secretion of gonadotropin-releasing hormone (GnRH). Early activation of the hypothalamic-pituitary-gonadal axis results in central precocious puberty. The timing of pubertal development is driven in part by genetic factors, but only a few, rare molecular defects associated with central precocious puberty have been identified.
Methods: We performed whole-exome sequencing in 40 members of 15 families with central precocious puberty. Candidate variants were confirmed with Sanger sequencing. We also performed quantitative real-time polymerase-chain-reaction assays to determine levels of messenger RNA (mRNA) in the hypothalami of mice at different ages.
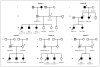
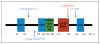
Results: We identified four novel heterozygous mutations in MKRN3, the gene encoding makorin RING-finger protein 3, in 5 of the 15 families; both sexes were affected. The mutations included three frameshift mutations, predicted to encode truncated proteins, and one missense mutation, predicted to disrupt protein function. MKRN3 is a paternally expressed, imprinted gene located in the Prader-Willi syndrome critical region (chromosome 15q11-q13). All affected persons inherited the mutations from their fathers, a finding that indicates perfect segregation with the mode of inheritance expected for an imprinted gene. Levels of Mkrn3 mRNA were high in the arcuate nucleus of prepubertal mice, decreased immediately before puberty, and remained low after puberty.
Conclusions: Deficiency of MKRN3 causes central precocious puberty in humans. (Funded by the National Institutes of Health and others.).
Figures

Comment in
-
Releasing the brake on puberty.N Engl J Med. 2013 Jun 27;368(26):2513-5. doi: 10.1056/NEJMe1306743. Epub 2013 Jun 5. N Engl J Med. 2013. PMID: 23738512 No abstract available.
-
Reproductive endocrinology: novel mutations linked to central precocious puberty.Nat Rev Endocrinol. 2013 Aug;9(8):440. doi: 10.1038/nrendo.2013.127. Epub 2013 Jun 25. Nat Rev Endocrinol. 2013. PMID: 23797819 No abstract available.
References
-
- Terasawa E, Fernandez DL. Neurobio-logical mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–51. - PubMed
-
- Gajdos ZK, Hirschhorn JN, Palmert MR. What controls the timing of puberty? An update on progress from genetic investigation. Curr Opin Endocrinol Diabetes Obes. 2009;16:16–24. - PubMed
-
- Palmert MR, Boepple PA. Variation in the timing of puberty: clinical spectrum and genetic investigation. J Clin Endocrinol Metab. 2001;86:2364–8. - PubMed
-
- Golub MS, Collman GW, Foster PM, et al. Public health implications of altered puberty timing. Pediatrics. 2008;121(Suppl 3):S218–S230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K99 HD071970/HD/NICHD NIH HHS/United States
- K23 HD073351/HD/NICHD NIH HHS/United States
- R00 HD071970/HD/NICHD NIH HHS/United States
- P30 HD018655/HD/NICHD NIH HHS/United States
- 1K23HD073351/HD/NICHD NIH HHS/United States
- F05 HD072773/HD/NICHD NIH HHS/United States
- 1F05HD072773-01/HD/NICHD NIH HHS/United States
- R21HD066495/HD/NICHD NIH HHS/United States
- R21 HD066495/HD/NICHD NIH HHS/United States
- U54HD28138/HD/NICHD NIH HHS/United States
- T32 DK007529/DK/NIDDK NIH HHS/United States
- K12 HD051959/HD/NICHD NIH HHS/United States
- U54 HD028138/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
