A high-throughput functional screen identifies small molecule regulators of temperature- and mechano-sensitive K2P channels
- PMID: 23738709
- PMCID: PMC3747594
- DOI: 10.1021/cb400289x
A high-throughput functional screen identifies small molecule regulators of temperature- and mechano-sensitive K2P channels
Abstract
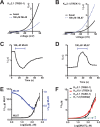
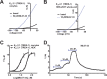
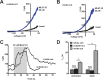
K2P (KCNK) potassium channels generate "leak" potassium currents that strongly influence cellular excitability and contribute to pain, somatosensation, anesthesia, and mood. Despite their physiological importance, K2Ps lack specific pharmacology. Addressing this issue has been complicated by the challenges that the leak nature of K2P currents poses for electrophysiology-based high-throughput screening strategies. Here, we present a yeast-based high-throughput screening assay that avoids this problem. Using a simple growth-based functional readout, we screened a library of 106,281 small molecules and identified two new inhibitors and three new activators of the mammalian K2P channel K2P2.1 (KCNK2, TREK-1). By combining biophysical, structure-activity, and mechanistic analysis, we developed a dihydroacridine analogue, ML67-33, that acts as a low micromolar, selective activator of temperature- and mechano-sensitive K2P channels. Biophysical studies show that ML67-33 reversibly increases channel currents by activating the extracellular selectivity filter-based C-type gate that forms the core gating apparatus on which a variety of diverse modulatory inputs converge. The new K2P modulators presented here, together with the yeast-based assay, should enable both mechanistic and physiological studies of K2P activity and facilitate the discovery and development of other K2P small molecule modulators.
Figures




References
-
- Enyedi P.; Czirjak G. (2010) Molecular background of leak K+ currents: two-pore domain potassium channels. Physiol. Rev. 90, 559–605. - PubMed
-
- Lesage F.; Barhanin J. (2011) Molecular physiology of pH-sensitive background K(2P) channels. Physiology 26, 424–437. - PubMed
-
- Es-Salah-Lamoureux Z.; Steele D. F.; Fedida D. (2010) Research into the therapeutic roles of two-pore-domain potassium channels. Trends Pharmacol. Sci. 31, 587–595. - PubMed
-
- Lotshaw D. P. (2007) Biophysical, pharmacological, and functional characteristics of cloned and native mammalian two-pore domain K+ channels. Cell Biochem. Biophys. 47, 209–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

