EEG and epilepsy monitoring
- PMID: 23739100
- PMCID: PMC10563901
- DOI: 10.1212/01.CON.0000431378.51935.d8
EEG and epilepsy monitoring
Abstract
Purpose of review: This article reviews the utility of EEG and prolonged video-EEG telemetry in the diagnosis and management of a patient with epilepsy.
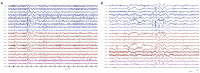
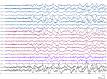
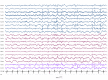
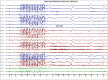
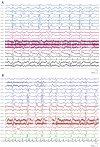
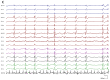
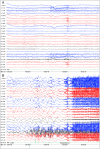
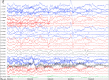
Recent findings: The EEG can be the most helpful test to determine a diagnosis of epilepsy; it can also distinguish focal and generalized neurophysiologic correlates of epilepsy. Furthermore, when paired with video monitoring, EEG can not only define epileptic and nonepileptic events but also aid in localization of seizures in patients with epilepsy. Finally, when history and other imaging modalities are considered with the EEG, the epileptic syndrome can usually be defined and the treatment can be focused. In critically ill patients, continuous EEG monitoring can define subclinical seizures, although a variety of periodic patterns may also be identified.
Summary: EEG is an invaluable tool in the diagnosis and management of a patient with epilepsy, and continuous EEG monitoring is useful in identifying subclinical seizures and nonconvulsive status epilepticus in critically ill patients.
Figures








References
-
- Tao JX,, Ray A,, Hawes-Ebersole S,, Ebersole JS. Intracranial substrates of scalp EEG spikes. Epilepsia 2005; 46 (5): 669–676. - PubMed
-
- Devinsky O,, Sato S,, Kufta CV, et al. Electroencephalographic studies of simple partial seizures with subdural electrode recordings. Neurology 1989; 39 (4): 527–533. - PubMed
-
- McCormick DA,, Contreras D. On the cellular and network bases of epileptic seizures. Ann Rev Physiol 2001; 63: 815–846. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
