An exclusively mesodermal origin of fin mesenchyme demonstrates that zebrafish trunk neural crest does not generate ectomesenchyme
- PMID: 23739134
- PMCID: PMC3699280
- DOI: 10.1242/dev.093534
An exclusively mesodermal origin of fin mesenchyme demonstrates that zebrafish trunk neural crest does not generate ectomesenchyme
Abstract
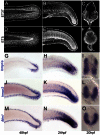
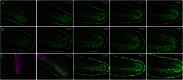
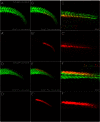
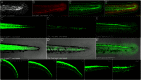
The neural crest is a multipotent stem cell population that arises from the dorsal aspect of the neural tube and generates both non-ectomesenchymal (melanocytes, peripheral neurons and glia) and ectomesenchymal (skeletogenic, odontogenic, cartilaginous and connective tissue) derivatives. In amniotes, only cranial neural crest generates both classes, with trunk neural crest restricted to non-ectomesenchyme. By contrast, it has been suggested that anamniotes might generate derivatives of both classes at all axial levels, with trunk neural crest generating fin osteoblasts, scale mineral-forming cells and connective tissue cells; however, this has not been fully tested. The cause and evolutionary significance of this cranial/trunk dichotomy, and its absence in anamniotes, are debated. Recent experiments have disputed the contribution of fish trunk neural crest to fin osteoblasts and scale mineral-forming cells. This prompted us to test the contribution of anamniote trunk neural crest to fin connective tissue cells. Using genetics-based lineage tracing in zebrafish, we find that these fin mesenchyme cells derive entirely from the mesoderm and that neural crest makes no contribution. Furthermore, contrary to previous suggestions, larval fin mesenchyme cells do not generate the skeletogenic cells of the adult fin, but persist to form fibroblasts associated with adult fin rays. Our data demonstrate that zebrafish trunk neural crest does not generate ectomesenchymal derivatives and challenge long-held ideas about trunk neural crest fate. These findings have important implications for the ontogeny and evolution of the neural crest.
Keywords: Dermomyotome; Ectomesenchyme; Fibroblast; Fin mesenchyme; Neural crest.
Figures


References
-
- Abzhanov A., Tzahor E., Lassar A. B., Tabin C. J. (2003). Dissimilar regulation of cell differentiation in mesencephalic (cranial) and sacral (trunk) neural crest cells in vitro. Development 130, 4567–4579 - PubMed
-
- Baroffio A., Dupin E., Le Douarin N. M. (1991). Common precursors for neural and mesectodermal derivatives in the cephalic neural crest. Development 112, 301–305 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

