NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy
- PMID: 23739234
- PMCID: PMC3772982
- DOI: 10.1038/ki.2013.207
NALP3-mediated inflammation is a principal cause of progressive renal failure in oxalate nephropathy
Abstract
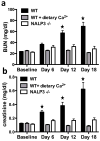
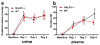
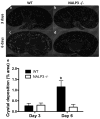
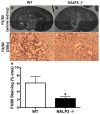
Oxalate nephropathy with renal failure is caused by multiple disorders leading to hyperoxaluria due to either overproduction of oxalate (primary hyperoxaluria) or excessive absorption of dietary oxalate (enteric hyperoxaluria). To study the etiology of renal failure in crystal-induced kidney disease, we created a model of progressive oxalate nephropathy by feeding mice a diet high in soluble oxalate (high oxalate in the absence of dietary calcium). Renal histology was characterized by intratubular calcium-oxalate crystal deposition with an inflammatory response in the surrounding interstitium. Oxalate nephropathy was not found in mice fed a high oxalate diet that also contained calcium. NALP3, also known as cryopyrin, has been implicated in crystal-associated diseases such as gout and silicosis. Mice fed the diet high in soluble oxalate demonstrated increased NALP3 expression in the kidney. Nalp3-null mice were completely protected from the progressive renal failure and death that occurred in wild-type mice fed the diet high in soluble oxalate. NALP3 deficiency did not affect oxalate homeostasis, thereby excluding differences in intestinal oxalate handling to explain the observed phenotype. Thus, progressive renal failure in oxalate nephropathy results primarily from NALP3-mediated inflammation.
Figures
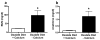

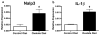

Comment in
-
A crystal-clear mechanism of chronic kidney disease.Kidney Int. 2013 Nov;84(5):859-61. doi: 10.1038/ki.2013.251. Kidney Int. 2013. PMID: 24172728
References
-
- Hoppe B, Langman CB. A United States survey on diagnosis, treatment, and outcome of primary hyperoxaluria. Pediatr Nephrol. 2003;18:986–991. - PubMed
-
- Lefaucheur C, Nochy D, Amrein C, et al. Renal histopathological lesions after lung transplantation in patients with cystic fibrosis. Am J Tranplantation. 2008;8:1901–1910. - PubMed
-
- Mandell I, Krauss E, Millan JC. Oxalate-induced acute renal failure in Crohn’s disease. Am J Med. 1980;69:628–632. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

