Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation
- PMID: 23740229
- PMCID: PMC3883901
- DOI: 10.1136/annrheumdis-2012-202925
Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation
Abstract
Objectives: Interleukin (IL)-23 has been implicated in the pathogenesis of ankylosing spondylitis (AS). The aim of the study was to clarify the mechanisms underlying the increased IL-23 expression in the gut of AS patients.
Methods: Consecutive gut biopsies from 30 HLA-B27(+) AS patients, 15 Crohn's disease (CD) patients and 10 normal subjects were obtained. Evidence for HLA-B27 misfolding was studied. Unfolded protein response (UPR) and autophagy were assessed by RT-PCR and immunohistochemistry. The contribution of UPR and autophagy in the regulation of IL-23 expression was evaluated in in vitro experiments on isolated lamina propria mononuclear cells (LPMCs).
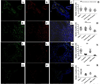
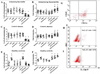
Results: Intracellular colocalisation of SYVN1 and FHCs but not a significant overexpression of UPR genes was observed in the gut of AS patients. Conversely, upregulation of the genes involved in the autophagy pathway was observed in the gut of AS and CD patients. Immunohistochemistry showed an increased expression of LC3II, ATG5 and ATG12 but not of SQSTM1 in the ileum of AS and CD patients. LC3II was expressed among infiltrating mononuclear cells and epithelial cells resembling Paneth cells (PC) and colocalised with ATG5 in AS and CD. Autophagy but not UPR was required to modulate the expression of IL-23 in isolated LPMCs of AS patients with chronic gut inflammation, CD patients and controls.
Conclusions: Our data suggest that HLA-B27 misfolding occurs in the gut of AS patients and is accompanied by activation of autophagy rather than a UPR. Autophagy appears to be associated with intestinal modulation of IL-23 in AS.
Keywords: Cytokines; Inflammation; Spondyloarthritis.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://group.bmj.com/group/rights-licensing/permissions.
Figures



Comment in
-
IL-23 expression and activation of autophagy in synovium and PBMCs of HLA-B27 positive patients with ankylosing spondylitis. Response to: 'Evidence that autophagy, but not the unfolded protein response, regulates the expression of IL-23 in the gut of patients with ankylosing spondylitis and subclinical gut inflammation' by Ciccia et al.Ann Rheum Dis. 2014 Nov;73(11):e68. doi: 10.1136/annrheumdis-2014-206277. Epub 2014 Jul 29. Ann Rheum Dis. 2014. PMID: 25074689 No abstract available.
References
-
- Mielants H, Veys EM, Cuvelier C, de Vos M. Ileocolonoscopic findings in seronegative spondylarthropathies. Br J Rheumatol. 1988;27:95–105. - PubMed
-
- Mielants H, Veys EM, De Vos M, et al. The evolution of spondyloarthropathies in relation to gut histology. I. Clinical aspects. J Rheumatol. 1995;22:2266–2272. - PubMed
-
- Mielants H, Veys EM, Cuvelier C, et al. The evolution of spondyloarthropathies in relation to gut histology. II. Histological aspects. J Rheumatol. 1995;22:2273–2278. - PubMed
-
- Ciccia F, Bombardieri M, Principato A, et al. Overexpression of interleukin-23, but not interleukin-17, as an immunologic signature of subclinical intestinal inflammation in ankylosing spondylitis. Arthritis Rheum. 2009;60:955–965. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

