Maintenance of biologic-free remission with naproxen or no treatment in patients with early, active axial spondyloarthritis: results from a 6-month, randomised, open-label follow-up study, INFAST Part 2
- PMID: 23740231
- PMCID: PMC3888608
- DOI: 10.1136/annrheumdis-2013-203460
Maintenance of biologic-free remission with naproxen or no treatment in patients with early, active axial spondyloarthritis: results from a 6-month, randomised, open-label follow-up study, INFAST Part 2
Abstract
Objective: To investigate whether biologic-free remission can be achieved in patients with early, active axial spondyloarthritis (SpA) who were in partial remission after 28 weeks of infliximab (IFX)+naproxen (NPX) or placebo (PBO)+NPX treatment and whether treatment with NPX was superior to no treatment to maintain disease control.
Method: Infliximab as First-Line Therapy in Patients with Early Active Axial Spondyloarthritis Trial (INFAST) Part 1 was a double-blind, randomised, controlled trial in biologic-naïve patients with early, active, moderate-to-severe axial SpA treated with either IFX 5 mg/kg+NPX 1000 mg/d or PBO+NPX 1000 mg/d for 28 weeks. Patients achieving Assessment of SpondyloArthritis international Society (ASAS) partial remission at week 28 continued to Part 2 and were randomised (1:1) to NPX or no treatment until week 52. Treatment group differences in ASAS partial remission and other efficacy variables were assessed through week 52 with Fisher exact tests.
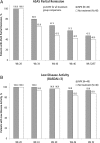
Results: At week 52, similar percentages of patients in the NPX group (47.5%, 19/40) and the no-treatment group (40.0%, 16/40) maintained partial remission, p=0.65. Median duration of partial remission was 23 weeks in the NPX group and 12.6 weeks in the no-treatment group (p=0.38). Mean Bath Ankylosing Spondylitis Disease Activity Index scores were low at week 28, the start of follow-up treatment (NPX, 0.7; no treatment, 0.6), and remained low at week 52 (NPX, 1.2; no treatment, 1.7).
Conclusions: In axial SpA patients who reached partial remission after treatment with either IFX+NPX or NPX alone, disease activity remained low, and about half of patients remained in remission during 6 months in which NPX was continued or all treatments were stopped.
Keywords: NSAIDs; Spondyloarthritis; TNF-alpha.
Figures



References
-
- van der Heijde D, Sieper J, Maksymowych WP, et al. Assessment of SpondyloArthritis international Society 2010 Update of the international ASAS recommendations for the use of anti-TNF agents in patients with axial spondyloarthritis. Ann Rheum Dis 2011;70:905–8 - PubMed
-
- Davis JC, Jr, van der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum 2003;48:3230–6 - PubMed
-
- van der Heijde D, Dijkmans B, Geusens P, et al. Efficacy and safety of infliximab in patients with ankylosing spondylitis: results of a randomized, placebo-controlled trial (ASSERT). Arthritis Rheum 2005;52:582–91 - PubMed
-
- Haibel H, Rudwaleit M, Listing J, et al. Efficacy of adalimumab in the treatment of axial spondyloarthritis without radiographically defined sacroiliitis: results of a twelve-week randomized, double-blind, placebo-controlled trial followed by an open-label extension up to week fifty-two. Arthritis Rheum 2008;58:1981–91 - PubMed
-
- Barkham N, Keen H, Coates L, et al. Clinical and imaging efficacy of infliximab in HLA-B27-positive patients with magnetic resonance imaging-determined early sacroiliitis. Arthritis Rheum 2009;60:946–54 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

