An undesired effect of chemotherapy: gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor κB- and hypoxia-inducible factor 1α-mediated up-regulation of CXCR4
- PMID: 23740244
- PMCID: PMC3774385
- DOI: 10.1074/jbc.M113.484576
An undesired effect of chemotherapy: gemcitabine promotes pancreatic cancer cell invasiveness through reactive oxygen species-dependent, nuclear factor κB- and hypoxia-inducible factor 1α-mediated up-regulation of CXCR4
Abstract
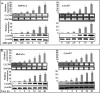
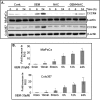
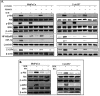
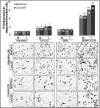
Recently, we have shown that CXCL12/CXCR4 signaling plays an important role in gemcitabine resistance of pancreatic cancer (PC) cells. Here, we explored the effect of gemcitabine on this resistance mechanism. Our data demonstrate that gemcitabine induces CXCR4 expression in two PC cell lines (MiaPaCa and Colo357) in a dose- and time-dependent manner. Gemcitabine-induced CXCR4 expression is dependent on reactive oxygen species (ROS) generation because it is abrogated by pretreatment of PC cells with the free radical scavenger N-acetyl-L-cysteine. CXCR4 up-regulation by gemcitabine correlates with time-dependent accumulation of NF-κB and HIF-1α in the nucleus. Enhanced binding of NF-κB and HIF-1α to the CXCR4 promoter is observed in gemcitabine-treated PC cells, whereas their silencing by RNA interference causes suppression of gemcitabine-induced CXCR4 expression. ROS induction upon gemcitabine treatment precedes the nuclear accumulation of NF-κB and HIF-1α, and suppression of ROS diminishes these effects. The effect of ROS on NF-κB and HIF-1α is mediated through activation of ERK1/2 and Akt, and their pharmacological inhibition also suppresses gemcitabine-induced CXCR4 up-regulation. Interestingly, our data demonstrate that nuclear accumulation of NF-κB results from phosphorylation-induced degradation of IκBα, whereas HIF-1α up-regulation is NF-κB-dependent. Lastly, our data demonstrate that gemcitabine-treated PC cells are more motile and exhibit significantly greater invasiveness against a CXCL12 gradient. Together, these findings reinforce the role of CXCL12/CXCR4 signaling in gemcitabine resistance and point toward an unintended and undesired effect of chemotherapy.
Keywords: CXCR4; Chromatography; Hypoxia-inducible Factor (HIF); NF-κB; Pancreatic Cancer; Reactive Oxygen Species (ROS).
Figures



References
-
- Siegel R., Naishadham D., Jemal A. (2013) Cancer statistics, 2013. CA-Cancer J. Clin. 63, 11–30 - PubMed
-
- Bardeesy N., DePinho R. A. (2002) Pancreatic cancer biology and genetics. Nat. Rev. Cancer 2, 897–909 - PubMed
-
- Olive K. P., Jacobetz M. A., Davidson C. J., Gopinathan A., McIntyre D., Honess D., Madhu B., Goldgraben M. A., Caldwell M. E., Allard D., Frese K. K., Denicola G., Feig C., Combs C., Winter S. P., Ireland-Zecchini H., Reichelt S., Howat W. J., Chang A., Dhara M., Wang L., Rückert F., Grützmann R., Pilarsky C., Izeradjene K., Hingorani S. R., Huang P., Davies S. E., Plunkett W., Egorin M., Hruban R. H., Whitebread N., McGovern K., Adams J., Iacobuzio-Donahue C., Griffiths J., Tuveson D. A. (2009) Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 - PMC - PubMed
-
- Wang Z., Li Y., Ahmad A., Banerjee S., Azmi A. S., Kong D., Sarkar F. H. (2011) Pancreatic cancer. Understanding and overcoming chemoresistance. Nat. Rev. Gastroenterol. Hepatol. 8, 27–33 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

