The minimum M3-M4 loop length of neurotransmitter-activated pentameric receptors is critical for the structural integrity of cytoplasmic portals
- PMID: 23740249
- PMCID: PMC3724616
- DOI: 10.1074/jbc.M113.481689
The minimum M3-M4 loop length of neurotransmitter-activated pentameric receptors is critical for the structural integrity of cytoplasmic portals
Abstract
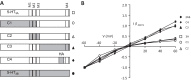
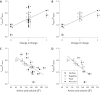
The 5-HT3A receptor homology model, based on the partial structure of the nicotinic acetylcholine receptor from Torpedo marmorata, reveals an asymmetric ion channel with five portals framed by adjacent helical amphipathic (HA) stretches within the 114-residue loop between the M3 and M4 membrane-spanning domains. The positive charge of Arg-436, located within the HA stretch, is a rate-limiting determinant of single channel conductance (γ). Further analysis reveals that positive charge and volume of residue 436 are determinants of 5-HT3A receptor inward rectification, exposing an additional role for portals. A structurally unresolved stretch of 85 residues constitutes the bulk of the M3-M4 loop, leaving a >45-Å gap in the model between M3 and the HA stretch. There are no additional structural data for this loop, which is vestigial in bacterial pentameric ligand-gated ion channels and was largely removed for crystallization of the Caenorhabditis elegans glutamate-activated pentameric ligand-gated ion channels. We created 5-HT3A subunit loop truncation mutants, in which sequences framing the putative portals were retained, to determine the minimum number of residues required to maintain their functional integrity. Truncation to between 90 and 75 amino acids produced 5-HT3A receptors with unaltered rectification. Truncation to 70 residues abolished rectification and increased γ. These findings reveal a critical M3-M4 loop length required for functions attributable to cytoplasmic portals. Examination of all 44 subunits of the human neurotransmitter-activated Cys-loop receptors reveals that, despite considerable variability in their sequences and lengths, all M3-M4 loops exceed 70 residues, suggesting a fundamental requirement for portal integrity.
Keywords: Cys-loop Receptors; GABA Receptors; Glycine Receptors; Ion Channels; Nicotinic Acetylcholine Receptors; Serotonin; Structural Biology.
Figures





References
-
- Bocquet N., Nury H., Baaden M., Le Poupon C., Changeux J. P., Delarue M., Corringer P. J. (2009) X-ray structure of a pentameric ligand-gated ion channel in an apparently open conformation. Nature 457, 111–114 - PubMed
-
- Hilf R. J., Dutzler R. (2008) X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 452, 375–379 - PubMed
-
- Niesler B., Frank B., Kapeller J., Rappold G. A. (2003) Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D, and HTR3E. Gene 310, 101–111 - PubMed
-
- Davies P. A., Pistis M., Hanna M. C., Peters J. A., Lambert J. J., Hales T. G., Kirkness E. F. (1999) The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 397, 359–363 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

