Abacavir-based triple nucleoside regimens for maintenance therapy in patients with HIV
- PMID: 23740608
- PMCID: PMC11330272
- DOI: 10.1002/14651858.CD008270.pub2
Abacavir-based triple nucleoside regimens for maintenance therapy in patients with HIV
Abstract
Background: Regimen simplification can be defined as a change in established effective therapy to reduce pill burden and dosing frequency, to enhance tolerability, or to decrease specific food and fluid requirements. Many patients on suppressive antiretroviral therapy may be considered candidates for a simplification strategy and, among them, those who have achieved virologic suppression. Several clinical trials have evaluated the efficacy of triple nucleoside combination as a simplification therapy in patients who achieved virologic suppression
Objectives: The aim of this review is to combine randomised, controlled trials to examine whether in patients with undetectable viraemia on a Protease inhibitor (PI) based regimen simplification treatment with abacavir (ABC)-based triple-nucleoside combinations has similar rates of efficacy and tolerability compared with a PI regimen or simplification with a NNRTIs (efavirenz-EFV- or nevirapine-NVP) containing regimen. Studies were included if they had at least two of the three interventions, including one 3NRTI arm.
Search methods: Electronic databases and conference proceedings were searched (1996-2012) with relevant search terms without limits to language.
Selection criteria: Randomised controlled trials (RCTs) only are included in this review. Patients population is represented by HIV-infected adult patients treated with a PI-containing regimen (PI or boosted PI), with undetectable viral load. Patients on a PI-containing regimen had three possibilities: continue the PI regimen or switch to a simplification maintenance regimen, including switch to a NNRTI (EFV or NVP) containing regimen, or switch to a triple-NRTI regimen (ABC-zidovudine-lamivudine)
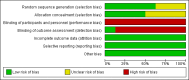
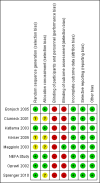
Data collection and analysis: The primary outcomes were: proportion of patients discontinuing or switching antiretroviral therapy due to virologic failure or to adverse events; death (all cause) and AIDS defining illness; occurrence of myocardial infarction and cardiovascular disease. Secondary outcomes were: proportion of patients maintaining an undetectable viral load (e.g. HIV-RNA <50 or <400 copies/mm(3)); change in mean CD4+ cell count; occurrence of lipodystrophy. We applied Cochrane Collaboration tools to assess each individual study for risk for bias.
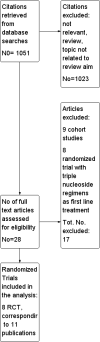
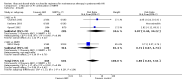
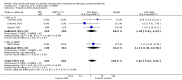
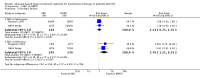
Main results: We included eight RCT, for a total of 1,610 patients. All the studies included HIV-1 infected patients virologically suppressed after a successful treatment with PI containing ART. Articles included in the analysis were published between 2001 and 2010, and could be classified as low risk of bias trials in most of the domains considered. Overall, there was no significant difference between the participants on triple nucleoside combination and controls, either PI-based or NNRTI based in terms of overall failures, death and AIDS related events, and rates of patients with viral load below the detectability cut-off. For the outcomes discontinuation for adverse events and virologic failures, the RRs were not significant , albeit being not far from the alpha level of 0.05, thus suggesting a weak evidence of lower incidence of side effects and an higher incidence of virologic failure in the 3NRTI group compared to controls . Change in lipids and in CD4 cells from baselines were reported in 7 studies, but inconsistency in reporting these data did not allow quantitative analysis. However, all agreed that simplification with ABC had a favourable and significant impact on lipid metabolism compared to control group. An increase in CD4 cells count from baseline was evident in all analysed studies, without significant differences between ABC and controls in individual studies.
Authors' conclusions: The strategy of switching to triple nucleoside regimens shows weak evidence of lower incidence of side effects and a higher incidence of virologic failure in the 3NRTI group compared to controls. Simplification with 3NRTI holds the advantages of preserving other classes of antiretroviral drugs, to lower blood lipids, and to be cost effective and simple to administer.Thus, simplification with triple nucleoside regimens AZT + 3TC + ABC should be still considered for individuals who are unable to tolerate or have contraindications to NNRTI or PI based regimens. Additional data are needed on longer-term efficacy of triple NRTI regimens, particularly on the development of antiretroviral resistance. Though studies in the current review were conducted between 2001 and 2010, the large majority of patients from studies analysed received old PI regimens (e.g., indinavir, ritonavir, nelfinavir, saquinavir) not longer recommended by International Guidelines. Since current guidelines recommend new "lipid -friendly" PI, future studies should compare regimens containing these news PIs to triple NRTI regimens. More realistically, however, there are opportunities to examine these issues in existing cohorts.
Conflict of interest statement
MC: has been an advisor/consultant for Bayer, Cephalon and ViiV Health care, and has received honoraria for educational lectures from Abbott, ViiV Healthcare and Novartis. These activities were not related to his work with this review.
Figures

















Update of
- doi: 10.1002/14651858.CD008270
Similar articles
-
Co-formulated abacavir-lamivudine-zidovudine for initial treatment of HIV infection and AIDS.Cochrane Database Syst Rev. 2013 Mar 28;2013(3):CD005481. doi: 10.1002/14651858.CD005481.pub3. Cochrane Database Syst Rev. 2013. PMID: 23543540 Free PMC article.
-
Efavirenz or nevirapine in three-drug combination therapy with two nucleoside or nucleotide-reverse transcriptase inhibitors for initial treatment of HIV infection in antiretroviral-naïve individuals.Cochrane Database Syst Rev. 2016 Dec 10;12(12):CD004246. doi: 10.1002/14651858.CD004246.pub4. Cochrane Database Syst Rev. 2016. PMID: 27943261 Free PMC article.
-
Optimisation of antiretroviral therapy in HIV-infected children under 3 years of age.Cochrane Database Syst Rev. 2014 May 22;2014(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. Cochrane Database Syst Rev. 2014. PMID: 24852077 Free PMC article.
-
Antiretroviral therapy (ART) for treating HIV infection in ART-eligible pregnant women.Cochrane Database Syst Rev. 2010 Mar 17;2010(3):CD008440. doi: 10.1002/14651858.CD008440. Cochrane Database Syst Rev. 2010. PMID: 20238370 Free PMC article.
-
Effectiveness of antiretroviral therapy in HIV-infected children under 2 years of age.Cochrane Database Syst Rev. 2012 Jul 11;(7):CD004772. doi: 10.1002/14651858.CD004772.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2014 May 22;(5):CD004772. doi: 10.1002/14651858.CD004772.pub4. PMID: 22786492 Updated.
Cited by
-
Longitudinal virological outcomes and factors associated with virological failure in behaviorally HIV-infected young adults on combination antiretroviral treatment in the Netherlands, 2000 to 2015.Medicine (Baltimore). 2019 Aug;98(32):e16357. doi: 10.1097/MD.0000000000016357. Medicine (Baltimore). 2019. PMID: 31393344 Free PMC article.
-
Antiviral efficacy and safety of abacavir-containing combination antiretroviral therapy as first-line treatment of HIV-infected children and adolescents: a systematic review protocol.Syst Rev. 2014 Aug 12;3:87. doi: 10.1186/2046-4053-3-87. Syst Rev. 2014. PMID: 25115243 Free PMC article.
-
Modifying Antiretroviral Therapy in Virologically Suppressed HIV-1-Infected Patients.Drugs. 2016 Jan;76(1):75-98. doi: 10.1007/s40265-015-0515-6. Drugs. 2016. PMID: 26677129 Free PMC article. Review.
-
Efficacy and safety of abacavir-containing combination antiretroviral therapy as first-line treatment of HIV infected children and adolescents: a systematic review and meta-analysis.BMC Infect Dis. 2015 Oct 26;15:469. doi: 10.1186/s12879-015-1183-6. BMC Infect Dis. 2015. PMID: 26502899 Free PMC article.
-
Severe abacavir hypersensitivity reaction in a patient with human immunodeficiency virus infection: a case report.J Med Case Rep. 2022 Nov 8;16(1):407. doi: 10.1186/s13256-022-03647-6. J Med Case Rep. 2022. PMID: 36345015 Free PMC article.
References
References to studies included in this review
Bonjoch 2005 {published data only}
-
- Bonjoch A, Paredes R, Galvez J. Antiretroviral treatment simplification with 3 NRTIs or 2 NRTIs plus nevirapine in HIV‐1‐infected patients treated with successful first‐line HAART. J Acquir Immune Defic Syndr 2005;39:313‐6. - PubMed
Clumeck 2001 {published data only}
-
- Clumeck N, Goebel F, Rozenbaum W, Gerstoft J, Staszewski S, Montaner J, Johnson M, Gazzard B, Stone C, Athisegaran R, Moore S, CNA30017 Study Team. Simplification with ABC‐based triple nucleoside therapy vs continued PI‐based ART in HIV‐1‐infected patients with undetectable plasma HIV‐1 RNA (CNA300017). AIDS 2001;15(12):1517‐26. - PubMed
Katlama 2003 {published data only}
-
- Katlama C, Fenske S, Gazzard B, Lazzarin A, Clumeck N, Mallolas J, Lafeuillade A, Mamet JP, Beauvais L, AZL30002 European study team. TRIZAL study: switching from successful HAART to Trizivir (abacavir‐lamivudine‐zidovudine combination tablet): 48 weeks efficacy, safety and adherence results. HIV Medicine 2003;4(2):79‐86. - PubMed
Keiser 2005 {published data only}
Maggiolo 2003 {published data only}
-
- Maggiolo F, Ripamonti D, Ravasio L, et al. Outcome of 2 simplification strategies for the treatment of human immunodeficiency virus type 1 infection.. Clin Infect Dis 2003;37:41‐9. - PubMed
NEFA Study {published data only}
-
- Martínez E, Arnaiz JA, Podzamczer D, et al. Substitution of nevirapine, efavirenz, or abacavir for protease inhibitors in patients with human immunodeficiency virus infection. N Engl J Med. 2003;349:1036‐46. - PubMed
-
- Martínez E, Arnaiz JA, Podzamczer D, et al. Three‐year follow‐up of protease inhibitor‐based regimen simplification in HIV‐infected patients. AIDS. AIDS 2007;21:367‐9. - PubMed
-
- Ochoa de Echaguen A, Arnedo M, Xercavins M, et al. Genotypic and phenotypic resistance patterns at virological failure in a simplification trial with nevirapine, efavirenz or abacavir. AIDS 2005;19:1385‐91. - PubMed
-
- Fisac C, Fumero E, Crespo M, et al. Metabolic benefits 24 months after replacing a protease inhibitor with abacavir, efavirenz or nevirapine. AIDS 2005;19:917‐25. - PubMed
Opravil 2002 {published data only}
-
- Opravil M, Hirschel B, Lazzarin A, et al. A randomized trial of simplified maintenance therapy with abacavir, lamivudine, and zidovudine in human immunodeficiency virus infection. J Infect Dis 2002;185:1251‐60. - PubMed
Sprenger 2010 {published data only}
-
- Sprenger HG, Langebeek N, Mulder PG, et al. Abacavir/lamivudine/zidovudine maintenance after standard induction in antiretroviral therapy‐naïve patients: FREE randomized trial interim results. AIDS Patient Care STDS 2010;24:361‐6. - PubMed
References to studies excluded from this review
Abgrall 2006 {published data only}
-
- Abgrall S, Yeni PG, Bouchaud O, Costagliola D, Clinical Epidemiology Group from the French Hospital Database on HIV. Switch from a first virologically effective protease inhibitor‐containing regimen to a regimen containing efavirenz, nevirapine or abacavir. AIDS 2006;20:2099‐106. - PubMed
Abgrall 2007 {published data only}
-
- Abgrall S, Yeni PG, Bouchaud O, Costagliola D, Clinical Epidemiology Group of the French Hospital Database on HIV. Comparative biological and clinical outcomes after a switch from a virologically unsuccessful first protease inhibitor‐containing antiretroviral combination to a 3‐drug regimen containing efavirenz, nevirapine, or abacavir.. Clin Infect Dis 2007;44:120‐7. - PubMed
Bommenel 2011 {published data only}
-
- Bommenel T, Launay O, Meynard JL, et al. Comparative effectiveness of continuing a virologically effective first‐line boosted protease inhibitor combination or of switching to a three‐drug regimen containing either efavirenz, nevirapine or abacavir. J Antimicrob Chemother 2011;66:1869‐77. - PubMed
Chiesa 2003 {published data only}
-
- Chiesa E, Bini T, Adorni F, et al. Simplification of protease inhibitor‐containing regimens with efavirenz, nevirapine or abacavir: safety and efficacy outcomes. Antiviral Therapy 203;8:27‐35. - PubMed
Cozzi‐lepri 2006 {published data only}
-
- Cozzi‐Lepri A, Luca A, Phillips AN, et al. A comparison between abacavir and efavirenz as the third drug used in combination with a background therapy regimen of 2 nucleoside reverse‐transcriptase inhibitors in patients with initially suppressed viral loads. J Infect Dis 2006;194:20‐8. - PubMed
Gulick 2004 {published data only}
-
- Gulick RM, Ribaudo HJ, Shikuma CM, et al. Triple‐nucleoside regimens versus efavirenz‐containing regimens for the initial treatment of HIV‐1 infection.. N Engl J Med 2004;350(18):1850‐61. - PubMed
Ibarra Barrueta 2004 {published data only}
-
- Ibarra Barrueta O, Martínez Bengoechea MJ, Illaro Uranga A, Lertxundi Etxebarría U, Iglesias Lambarri A, Santos Ibáñez A. Simplification to lamivudine, zidovudine, and abacavir therapy: impact on adherence, clinical outcome, and economic issues [Simplificación con lamivudina, zidovudina y abacavir:repercusión sobre la adherencia, resultados clínicos e impacto económico]. Farmacia Hospitalaria 2004;28(Suppl. 1):27‐33. - PubMed
Kumar 2006 {published data only}
-
- Kumar PN, Rodriguez‐French A, Thompson MA, et al. A prospective, 96‐week study of the impact of Trizivir, Combivir/nelfinavir, and lamivudine/stavudine/nelfinavir on lipids, metabolic parameters and efficacy in antiretroviral‐naive patients: effect of sex and ethnicity.. HIV Medicine 2006;7(2):85‐98. - PubMed
Kumar 2009 {published data only}
-
- Kumar PN, Salvato P, Lamarca A, Dejesus E, Patel P, McClernon D, et al. A randomized, controlled trial of initial anti‐retroviral therapy with abacavir/lamivudine/zidovudine twice‐daily compared to atazanavir once‐daily with lamivudine/zidovudine twice‐daily in HIV‐infected patients over 48 weeks (ESS100327, the ACTION Study). AIDS Res Ther 2009;6:3. - PMC - PubMed
Matheron 2003 {published data only}
-
- Matheron S, Descamps D, Boué F, Livrozet JM, Lafeuillade A, Aquilina C, et al. CNA3007 Study Group. Triple nucleoside combination zidovudine/lamivudine/abacavir versus zidovudine/lamivudine/nelfinavir as first‐line therapy in HIV‐1‐infected adults: a randomized trial. Antivir Ther 2003;8:163‐71. - PubMed
Moyle 2002 {published data only}
-
- Moyle G, Gazzard BG. Switching to zidovudine plus lamivudine plus abacavir maintains viral suppression in patients with high viral load before antiretroviral therapy: a retrospective clinical cohort analysis. AIDS 2002;16(7):1086‐7. - PubMed
Munderi 2010 {published data only}
-
- Munderi P, Walker AS, Kityo C, et al. Nevirapine/zidovudine/lamivudine has superior immunological and virological responses not reflected in clinical outcomes in a 48‐week randomized comparison with abacavir/zidovudine/lamivudine in HIV‐infected Ugandan adults with low CD4 cell counts. HIV Medicine 2010;11:334‐44. - PubMed
Orkin 2004 {published data only}
-
- Orkin C, Stebbing J, Nelson M, Bower M, Johnson M, Mandalia S, et al. A randomized study comparing a three‐ and four‐drug HAART regimen in first‐line therapy (QUAD study). J Antimicrob Chemother 2005;55:246‐51. - PubMed
Palma 2007 {published data only}
-
- Palma P, Romiti ML, Cancrini C, et al. Successful simplification of protease inhibitor‐based HAART with triple nucleoside regimens in children vertically infected with HIV.. AIDS 2007;21:465‐72. - PubMed
Staszewsky 2001 {published data only}
-
- Staszewski S, Keiser P, Montaner J, et al. Abacavir‐lamivudine‐zidovudine vs indinavir‐lamivudine‐zidovudine in antiretroviral‐naive HIV‐infected adults: A randomized equivalence trial. JAMA 2001;285(9):1155‐63. - PubMed
Vibhagool 2004 {published data only}
-
- Vibhagool A, Cahn P, Schechter M, et al. Triple nucleoside treatment with abacavir plus the lamivudine/zidovudine combination tablet (COM) compared to indinavir/COM in antiretroviral therapy‐naive adults: results of a 48‐week open‐label, equivalence trial (CNA3014). Curr Med Res Opin 2004;20(7):1103‐14. - PubMed
Wolbers 2007 {published data only}
-
- Wolbers M, Opravil M, von Wyl V et al. Swiss HIV cohort study. Predictors of optimal viral suppression in patients switched to abacavir, lamivudine, and zidovudine: the Swiss HIV Cohort Study. AIDS 2007;21:2201‐7. - PubMed
Additional references
Arribas 2009
-
- Arribas JR, Delgado R, Arranz A, Mun˜oz R, Portilla J, et al. Lopinavir‐ritonavir monotherapyversus lopinavir‐ritonavir and 2 nucleosidesfor maintenance therapy of HIV: 96‐week analysis. J Acquir Immune Defic Syndr 2009;51:147‐52. - PubMed
Bedimo
-
- Bedimo R, Westfall A, Drechsler H, Tebas P. Abacavir use and risk of acute myocardial infarction and cerebrovascular disease in the HAART era. IAS 2009, Cape Town, 19‐22 July 2009. 2009:Abst. MOAB202.
Brothers 2009
-
- Brothers CH, Hernandez JE, Cutrell AG, et al. Risk of Myocardial Infarction and Abacavir therapy: No increased risk across 52 Glaxo‐Smith Kline‐sponsored clinical trials in adult subjects. Journal of the Acquired Immune Deficiency Syndrome. 2009;51:20‐8. - PubMed
Bucher 2003
-
- Bucher HC, Kofler A, Nüesch R, Young J, Battegay M, Opravil M. A Meta‐analysis of randomized controlled trials of simplified versus continued protease inhibitor‐based antiretroviral therapy in HIV‐1‐infected patients.. AIDS 2003;17(17):2451‐9. - PubMed
Cruciani 2011
-
- Cruciani M, Zanichelli V, Serpelloni G, et al. Abacavir Use and Cardiovascular Disease Events: a Meta‐analysis of Published and unpublished Data. AIDS 2011;25:1993‐2004. - PubMed
DAD 2007
-
- The DAD Study Group. Class of antiretroviral drugs and the risk of myocardial infarction. New England Journal of Medicine 2007;356(17):1723‐35. - PubMed
DAD 2008
DART 2006
-
- DART Virology Group and Trial Team. Virological response to a triple nucleoside/nucleotide analogue regimen over 48weeks in HIV‐1‐infected adults in Africa. AIDS. Jun 26 2006;20(10):1391‐1399.. AIDS 2006;20:1391‐9. - PubMed
DHHS 2008
-
- Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV‐1‐infected adults and adolescents.. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf November 23, 2008.
DHHS 2011
-
- Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV‐1‐infected adults and adolescents. www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf January 2011.
Duvivier 2009
-
- Duvivier C, Kolta S, Assoumou L, Ghosn J, Rozenberg S, Murphy RL, Katlama C, Costagliola D, ANRS 121 Hippocampe study group. Greater decrease in bone mineral density with protease inhibitor regimens compared with nonnucleoside reverse transcriptase inhibitor regimens in HIV‐1 infected naive patients. AIDS 2009;23:817‐24. - PubMed
EACS 2009
-
- European AIDS Clinical Society. Guidelines. Clinical management and treatment of HIV infected Adults in Europe . http://www.europeanaidsclinicalsociety.org/guidelines.asp November 2009.
Egger 1997
Higgins 2003
Higgins 2008
-
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.0. The Cochrane Collaboration. Available from www.Cochrane‐handbook.org 2008, issue updated February 2008.
Higgins 2009
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. [updated September 2008]. Available from www.cochrane‐handbook.org 2009. The Cochrane Collaboration., 2009.
Higgins 2011
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011., March 2011.
Katlama 2010
-
- Katlama C, Valantin MA, Algarte‐Genin M, Duvivier C, Lambert‐Niclot S, Girard PM, Molina JM, Hoen B, Pakianather S, Peytavin G, Marcelin AG, Flandre P. Efficacy of darunavir/ritonavir maintenance monotherapy in patients with HIV‐1 viral suppression: a randomized open‐label, noninferiority trial, MONOI‐ANRS 136. AIDS 2010;24:2365‐74. - PubMed
Kessler 2005
Lambert‐Niclot 2012
-
- Lambert‐Niclot, Flandre P, Valantin MA, Soulie C, Fourati, Wirden M, Sayon, Pakianather, Bocket L, Masquelier B, Dos Santos G, Katlama C, Calvez V, Marcelin AG. Similar evolution of cellular HIV‐1 DNA level in darunavir/ritonavir monotherapy versus triple therapy in MONOI‐ANRS136 trial over 96 weeks.. PLoS One 2012;7:e41390. - PMC - PubMed
Lang 2009
-
- Lang S, Mary‐Krause M, Cotte L, et al. Impact of specific NRTI and PI exposure on the risk of myocardial infarction: a case‐control study nested within FHDH ANRS CO4. 16th Conference on Retroviruses and Opportunistic Infections (CROI), Montreal, abstract 43LB, 2009.. 2009.
Lundgren 2009
-
- Lundgren J, Reiss P, Worm S, et al. Risk of miocardial infarction with exposure to specific ARV from the PI, NNRTI, and NRTI drug classes: The D:A:D Study. Abstract of the 16th Conference on retrovirus and opportunistic Infection (CROI), Montreal, february 2009. Abst. 44LB. 2009.
Mallal 2008
-
- Mallal S, Phillips E, Carosi G, et al. HLA‐B*5701 Screening for hypersensitivity to abacavir. New England Journal of Medicine 2008;358:568‐79. - PubMed
Martínez 2003
-
- Martínez E, Arnaiz JA, Podzamczer D, et al. Substitution of nevirapine, efavirenz, or abacavir for protease inhibitors in patients with human immunodeficiency virus infection. N Engl J Med. 2003;349:1036‐46. - PubMed
Martínez 2007
-
- Martínez E, Arnaiz JA, Podzamczer D, et al. Three‐year follow‐up of protease inhibitor‐based regimen simplification in HIV‐infected patients. AIDS. AIDS 2007;21:367‐9. - PubMed
Ochoa de Echanguen 2005
-
- Ochoa de Echaguen A, Arnedo M, Xercavins M, et al. Genotypic and phenotypic resistance patterns at virological failure in a simplification trial with nevirapine, efavirenz or abacavir. AIDS 2005;19:1385‐91. - PubMed
Opravil 2004
-
- Opravil M, Baumann D, Chaveb JP, et al. Long‐term efficacy after switch from protease inhibitor‐containing highly active antiretroviral therapy toabacavir, lamivudine, and zidovudine. AIDS 2004;18:2213‐19. - PubMed
Pappa 2008
-
- Pappa K, Hernandez J, Ha B, et al. ABC/3TC shows robust virologic responses in ART‐naive patients for baseline (BL) viral loads of >100,000c/mL and <100,000c/mL by endpoint used in ACTG5202. XVII International AIDS Conference (AIDS 2008). Mexico City. August 3‐8, 2008. Abstract THAB0304. 2008.
RevMan 2011
-
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011., March 2011.
Ribaudo 2011
Rizzardini 2006
-
- Rizzardini G, Capetti A. Switch to abacavir‐based triple nucleoside regimens in HIV‐1 infected patients never treated with suboptimal antiretroviral therapy: a review. Medical Science Monitor 2006;12(12):269‐76. - PubMed
Sax 2008
-
- Sax P, Tierney C, Collier A, et al. ACTG 5202: shorter time to virologic failure with ABC/3TC than TENOFOVIR/FTC in treatment‐naive subjects with HIV RNA >100,000. XVII International AIDS Conference (AIDS 2008). Mexico City. August 3‐8, 2008. Abstract THAB0303. 2008.
SMART 2008
Smith 2008
-
- Smith KY, Fine D, Patel P, et al. Similarity in efficacy and safety of abacavir/lamivudine (ABC/3TC) compared to tenofovir/emtricitabine (TENOFOVIR/FTC) in combination with QD lopinavir/ritonavir (LPV/r) over 96 weeks in the HEAT study. XVII International AIDS Conference (AIDS 2008). Mexico City. August 3‐8, 2008. Abstract LBPE1138. 2008.
Valantin 2012
-
- Valantin MA, Lambert‐Niclot, Flandre P, Morand‐Joubert L, Cabiè A, Meynard JL, Ponscarme D, Ajana F, Slama L, Curjol A, Cuzin L, Schneider L, Taburet AM, Marcelin AG, Katlama C, MONOI ANRS 136 Study Group. Long‐term efficacy of darunavir/ritonavir monotherapy in patients with HIV‐1 viral suppression: week 96 results from the MONOI ANRS 136 study.. J Antimicrob Chemother 2012;67:691‐5. - PubMed
Ware 1993
-
- Ware JE Jr, Snow KK, Kosinski M, Gandek B. SF‐36 Health Survey manual and interpretation guide. Boston, Mass.: Nimrod Press, 1993.
WHO 2010
-
- World Health Organization. Antiretroviral therapy for HIV infection in adults and adolescents. Recommendations for a public health approach. 2010 revision. http://whqlibdoc.who.int/publications/2010/9789241599764_eng.pdf 2010. - PubMed
Worm 2010
-
- Worm SW, Sabin C, Weber R, Reiss P, El‐Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti‐HIV drugs (D:A:D) study. J Infect Dis 2010;201:318‐30. - PubMed
Wu 1997
-
- Wu AW, Hays RD, Kelly S, et al. Applications of the Medical Outcomes Study health‐related quality of life measures in HIV/AIDS.. Qual Life Res 1997;6:531‐4. - PubMed
Fisac 2005
-
- Fisac C, Fumero E, Crespo M, et al. Metabolic benefits 24 months after replacing a protease inhibitor with abacavir, efavirenz or nevirapine. AIDS 2005;19(917‐25). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

