High-throughput instant quantification of protein expression and purity based on photoactive yellow protein turn off/on label
- PMID: 23740751
- PMCID: PMC3810716
- DOI: 10.1002/pro.2286
High-throughput instant quantification of protein expression and purity based on photoactive yellow protein turn off/on label
Abstract
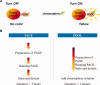
Quantifying the concentration and purity of a target protein is essential for high-throughput protein expression test and rapid screening of highly soluble proteins. However, conventional methods such as PAGE and dot blot assay generally involve multiple time-consuming tasks requiring hours or do not allow instant quantification. Here, we demonstrate a new method based on the Photoactive yellow protein turn Off/On Label (POOL) system that can instantly quantify the concentration and purity of a target protein. The main idea of POOL is to use Photoactive Yellow Protein (PYP), or its miniaturized version, as a fusion partner of the target protein. The characteristic blue light absorption and the consequent yellow color of PYP is absent when initially expressed without its chromophore, but can be turned on by binding its chromophore, p-coumaric acid. The appearance of yellow color upon adding a precursor of chromophore to the co-expressed PYP can be used to check the expression amount of the target protein via visual inspection within a few seconds as well as to quantify its concentration and purity with the aid of a spectrometer within a few minutes. The concentrations measured by the POOL method, which usually takes a few minutes, show excellent agreement with those by the BCA Kit, which usually takes ∼1 h. We demonstrate the applicability of POOL in E. coli, insect, and mammalian cells, and for high-throughput protein expression screening.
Keywords: color tag; high-throughput protein expression screening; protein expression test; protein purification; protein purity estimation; protein quantification.
© 2013 The Protein Society.
Figures




References
-
- Terpe K. Overview of tag protein fusions: from molecular and biochemical fundamentals to commercial systems. Appl Microbiol Biotechnol. 2003;60:523–533. - PubMed
-
- Jenny RJ, Mann KG, Lundblad RL. A critical review of the methods for cleavage of fusion proteins with thrombin and factor Xa. Protein Expr Purif. 2003;31:1–11. - PubMed
-
- Tykvart J, Sacha P, Barinka C, Knedlik T, Starkova J, Lubkowski J, Konvalinka J. Efficient and versatile one-step affinity purification of in vivo biotinylated proteins: expression, characterization and structure analysis of recombinant human glutamate carboxypeptidase II. Protein Expr Purif. 2012;82:106–115. - PMC - PubMed
-
- Shooltz DD, Alberts GL, Triezenberg SJ. One-step affinity purification of recombinant TATA binding proteins utilizing a modular protein interaction partner. Protein Expr Purif. 2008;59:297–301. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

