Structural transitions in tau k18 on micelle binding suggest a hierarchy in the efficacy of individual microtubule-binding repeats in filament nucleation
- PMID: 23740819
- PMCID: PMC3832040
- DOI: 10.1002/pro.2290
Structural transitions in tau k18 on micelle binding suggest a hierarchy in the efficacy of individual microtubule-binding repeats in filament nucleation
Abstract
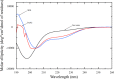
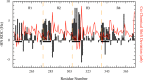
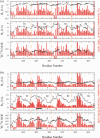
The protein tau is found in an aggregated filamentous state in the intraneuronal paired helical filament deposits characteristic of Alzheimer's disease and other related dementias and mutations in tau protein and mRNA cause frontotemproal dementia. Tau isoforms include a microtubule-binding domain containing either three or four imperfect tandem microtubule binding repeats that also form the core of tau filaments and contain hexapaptide motifs that are critical for tau aggregation. The tau microtubule-binding domain can also engage in direct interactions with detergents, fatty acids, or membranes, which can greatly facilitate tau aggregation and may also mediate some tau functions. Here, we show that the alternatively spliced second microtubule-binding repeat exhibits significantly different structural characteristics compared with the other three repeats in the context of the intact repeat domain. Most notably, the PHF6* hexapeptide motif located at the N-terminus of repeat 2 has a lower propensity to form strand-like structure than the corresponding PHF6 motif in repeat 3, and unlike PHF6 converts to partially helical structure in the micelle-bound state. Interestingly, the behavior of the Module-B motif, located at the beginning of repeat 4, resembles that of PHF6* rather than PHF6. Our observations, combined with previous results showing that PHF6* and Module-B are both less effective than PHF6 in nucleating tau aggregation, suggest a hierarchy in the efficacy of these motifs in nucleating tau aggregation that originates in differences in their intrinsic propensities for extended strand-like structure and the resistance of these propensities to changes in tau's environment.
Keywords: Alzheimer's; PHF6; PHF6*; amyloid; microtubule-binding domain; microtubule-binding repeat; paired helical filaments; protein aggregation; tau.
© 2013 The Protein Society.
Figures


References
-
- Cleveland DW, Hwo SY, Kirschner MW. Purification of tau, a microtubule-associated protein that induces assembly of microtubules from purified tubulin. J Mol Biol. 1977;116:207–225. - PubMed
-
- Cleveland DW, Hwo SY, Kirschner MW. Physical and chemical properties of purified tau factor and the role of tau in microtubule assembly. J Mol Biol. 1977;116:227–247. - PubMed
-
- Goedert M, Spillantini MG, Jakes R, Rutherford D, Crowther RA. Multiple isoforms of human microtubule-associated protein tau: sequences and localization in neurofibrillary tangles of Alzheimer's disease. Neuron. 1989;3:519–526. - PubMed
-
- Andreadis A, Brown WM, Kosik KS. Structure and novel exons of the human tau gene. Biochemistry. 1992;31:10626–10633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

