Dual receptor T cells mediate pathologic alloreactivity in patients with acute graft-versus-host disease
- PMID: 23740900
- PMCID: PMC3859344
- DOI: 10.1126/scitranslmed.3005452
Dual receptor T cells mediate pathologic alloreactivity in patients with acute graft-versus-host disease
Abstract
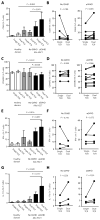
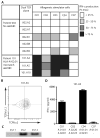
Acute graft-versus-host disease (aGVHD) results from a robust response of donor T cells transferred during hematopoietic stem cell transplantation (HSCT) to allogeneic peptide-major histocompatibility complex antigens. Previous investigations have not identified T cell subsets that selectively mediate either protective immunity or pathogenic alloreactivity. We demonstrate that the small subset of peripheral T cells that naturally express two T cell receptors (TCRs) on the cell surface contributes disproportionately to aGVHD in patients after allogeneic HSCT. Dual TCR T cells from patients with aGVHD demonstrate an activated phenotype and produce pathogenic cytokines ex vivo. Dual receptor clones from a patient with symptomatic aGVHD responded specifically to mismatched recipient human leukocyte antigens (HLAs), demonstrating pathologic alloreactivity. Human dual TCR T cells are strongly activated and expanded by allogeneic stimulation in vitro, and disproportionately contribute to the repertoire of T cells recognizing both major (HLA) and minor histocompatibility antigens, providing a mechanism for their observed activity in vivo in patients with aGVHD. These results identify dual TCR T cells as a target for focused analysis of a T cell subset mediating GVHD and as a potential prognostic indicator.
Conflict of interest statement
Figures


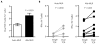

Comment in
-
One is better than two: TCR pairing and GVHD.Sci Transl Med. 2013 Jun 5;5(188):188fs21. doi: 10.1126/scitranslmed.3006431. Sci Transl Med. 2013. PMID: 23740895
Similar articles
-
Alloimmune T cells in transplantation.J Clin Invest. 2017 Jun 30;127(7):2473-2481. doi: 10.1172/JCI90595. Epub 2017 Jun 19. J Clin Invest. 2017. PMID: 28628037 Free PMC article. Review.
-
The highly alloreactive nature of dual TCR T cells.Curr Opin Organ Transplant. 2016 Feb;21(1):22-8. doi: 10.1097/MOT.0000000000000261. Curr Opin Organ Transplant. 2016. PMID: 26555233 Free PMC article. Review.
-
Analysis of the Whole CDR3 T Cell Receptor Repertoire after Hematopoietic Stem Cell Transplantation in 2 Clinical Cohorts.Biol Blood Marrow Transplant. 2020 Jun;26(6):1050-1070. doi: 10.1016/j.bbmt.2020.01.020. Epub 2020 Feb 18. Biol Blood Marrow Transplant. 2020. PMID: 32081787 Free PMC article. Clinical Trial.
-
Induction of alloanergy in human donor T cells without loss of pathogen or tumor immunity.Transplantation. 2008 Sep 27;86(6):854-64. doi: 10.1097/TP.0b013e3181861b6c. Transplantation. 2008. PMID: 18813111 Free PMC article.
-
Activation-associated phenotype of CD3 T cells in acute graft-versus-host disease.Clin Exp Immunol. 2006 Jul;145(1):36-43. doi: 10.1111/j.1365-2249.2006.03104.x. Clin Exp Immunol. 2006. PMID: 16792671 Free PMC article.
Cited by
-
Analysis of T-Cell Receptor Repertoire in Transplantation: Fingerprint of T Cell-mediated Alloresponse.Front Immunol. 2022 Jan 12;12:778559. doi: 10.3389/fimmu.2021.778559. eCollection 2021. Front Immunol. 2022. PMID: 35095851 Free PMC article. Review.
-
Alloimmune T cells in transplantation.J Clin Invest. 2017 Jun 30;127(7):2473-2481. doi: 10.1172/JCI90595. Epub 2017 Jun 19. J Clin Invest. 2017. PMID: 28628037 Free PMC article. Review.
-
The highly alloreactive nature of dual TCR T cells.Curr Opin Organ Transplant. 2016 Feb;21(1):22-8. doi: 10.1097/MOT.0000000000000261. Curr Opin Organ Transplant. 2016. PMID: 26555233 Free PMC article. Review.
-
The ability to rearrange dual TCRs enhances positive selection, leading to increased Allo- and Autoreactive T cell repertoires.J Immunol. 2014 Aug 15;193(4):1778-86. doi: 10.4049/jimmunol.1400532. Epub 2014 Jul 11. J Immunol. 2014. PMID: 25015825 Free PMC article.
-
A New Window into the Human Alloresponse.Transplantation. 2016 Aug;100(8):1639-49. doi: 10.1097/TP.0000000000001064. Transplantation. 2016. PMID: 26760572 Free PMC article. Review.
References
-
- Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7:340–352. - PubMed
-
- Bacigalupo A. Antilymphocyte/thymocyte globulin for graft versus host disease prophylaxis: Efficacy and side effects. Bone Marrow Transplant. 2005;35:225–231. - PubMed
-
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

