RD3 gene delivery restores guanylate cyclase localization and rescues photoreceptors in the Rd3 mouse model of Leber congenital amaurosis 12
- PMID: 23740938
- PMCID: PMC3766183
- DOI: 10.1093/hmg/ddt244
RD3 gene delivery restores guanylate cyclase localization and rescues photoreceptors in the Rd3 mouse model of Leber congenital amaurosis 12
Abstract
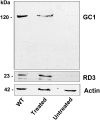
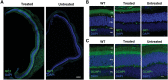
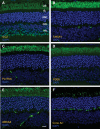
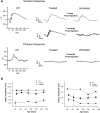
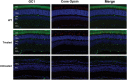
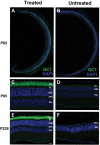
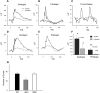
RD3 is a 23 kDa protein implicated in the stable expression of guanylate cyclase in photoreceptor cells. Truncation mutations are responsible for photoreceptor degeneration and severe early-onset vision loss in Leber congenital amaurosis 12 (LCA12) patients, the rd3 mouse and the rcd2 collie. To further investigate the role of RD3 in photoreceptors and explore gene therapy as a potential treatment for LCA12, we delivered adeno-associated viral vector (AAV8) with a Y733F capsid mutation and containing the mouse Rd3 complementary DNA (cDNA) under the control of the human rhodopsin kinase promoter to photoreceptors of 14-day-old Rb(11.13)4Bnr/J and In (5)30Rk/J strains of rd3 mice by subretinal injections. Strong RD3 transgene expression led to the translocation of guanylate cyclase from the endoplasmic reticulum (ER) to rod and cone outer segments (OSs) as visualized by immunofluorescence microscopy. Guanylate cyclase expression and localization coincided with the survival of rod and cone photoreceptors for at least 7 months. Rod and cone visual function was restored in the In (5)30Rk/J strain of rd3 mice as measured by electroretinography (ERG), but only rod function was recovered in the Rb(11.13)4Bnr/J strain, suggesting that the latter may have another defect in cone phototransduction. These studies indicate that RD3 plays an essential role in the exit of guanylate cyclase from the ER and its trafficking to photoreceptor OSs and provide a 'proof of concept' for AAV-mediated gene therapy as a potential therapeutic treatment for LCA12.
Figures


References
-
- den Hollander A.I., Roepman R., Koenekoop R.K., Cremers F.P. Leber congenital amaurosis: genes, proteins and disease mechanisms. Prog. Retin. Eye Res. 2008;27:391–419. - PubMed
-
- Perrault I., Rozet J.M., Calvas P., Gerber S., Camuzat A., Dollfus H., Chatelin S., Souied E., Ghazi I., Leowski C., et al. Retinal-specific guanylate cyclase gene mutations in Leber's congenital amaurosis. Nat. Genet. 1996;14:461–464. - PubMed
-
- Yang R.B., Garbers D.L. Two eye guanylyl cyclases are expressed in the same photoreceptor cells and form homomers in preference to heteromers. J. Biol. Chem. 1997;272:13738–13742. - PubMed
-
- Shyjan A.W., de Sauvage F.J., Gillett N.A., Goeddel D.V., Lowe D.G. Molecular cloning of a retina-specific membrane guanylyl cyclase. Neuron. 1992;9:727–737. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

