Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children
- PMID: 23741007
- PMCID: PMC3731936
- DOI: 10.1182/blood-2013-03-490219
Loss of endothelial protein C receptors links coagulation and inflammation to parasite sequestration in cerebral malaria in African children
Abstract
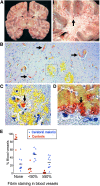
Cerebral malaria (CM) is a major cause of mortality in African children and the mechanisms underlying its development, namely how malaria-infected erythrocytes (IEs) cause disease and why the brain is preferentially affected, remain unclear. Brain microhemorrhages in CM suggest a clotting disorder, but whether this phenomenon is important in pathogenesis is debated. We hypothesized that localized cerebral microvascular thrombosis in CM is caused by a decreased expression of the anticoagulant and protective receptors thrombomodulin (TM) and endothelial protein C receptor (EPCR) and that low constitutive expression of these regulatory molecules in the brain make it particularly vulnerable. Autopsies from Malawian children with CM showed cerebral fibrin clots and loss of EPCR, colocalized with sequestered IEs. Using a novel assay to examine endothelial phenotype ex vivo using subcutaneous microvessels, we demonstrated that loss of EPCR and TM at sites of IE cytoadherence is detectible in nonfatal CM. In contrast, although clotting factor activation was seen in the blood of CM patients, this was compensated and did not disseminate. Because of the pleiotropic nature of EPCR and TM, these data implicate disruption of the endothelial protective properties at vulnerable sites and particularly in the brain, linking coagulation and inflammation with IE sequestration.
Figures




Comment in
-
The endothelial protein C receptor and malaria.Blood. 2013 Aug 1;122(5):624-5. doi: 10.1182/blood-2013-06-508531. Blood. 2013. PMID: 23908442
References
-
- Murray CJ, Rosenfeld LC, Lim SS, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet. 2012;379(9814):413–431. - PubMed
-
- Taylor TE, Fu WJ, Carr RA, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10(2):143–145. - PubMed
-
- Grau GE, Craig AG. Cerebral malaria pathogenesis: revisiting parasite and host contributions. Future Microbiol. 2012;7(2):291–302. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

