Physiological and anatomical properties of intramedullary projection neurons in rat rostral nucleus of the solitary tract
- PMID: 23741045
- PMCID: PMC3763094
- DOI: 10.1152/jn.00167.2013
Physiological and anatomical properties of intramedullary projection neurons in rat rostral nucleus of the solitary tract
Abstract
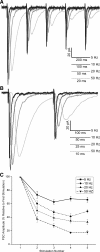
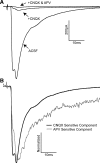
The rostral nucleus of the solitary tract (rNTS), the first-order relay of gustatory information, not only transmits sensory information to more rostral brain areas but also connects to various brain stem sites responsible for orofacial reflex activities. While much is known regarding ascending projections to the parabrachial nucleus, intramedullary projections to the reticular formation (which regulate oromotor reflexive behaviors) remain relatively unstudied. The present study examined the intrinsic firing properties of these neurons as well as their morphological properties and synaptic connectivity with primary sensory afferents. Using in vitro whole cell patch-clamp recording, we found that intramedullary projection neurons respond to depolarizing current injection with either tonic or bursting action potential trains and subsets of these groups of neurons express A-type potassium, H-like, and postinhibitory rebound currents. Approximately half of the intramedullary projection neurons tested received monosynaptic innervation from primary afferents, while the rest received polysynaptic innervation, indicating that at least a subpopulation of these neurons can be directly activated by incoming sensory information. Neuron morphological reconstructions revealed that many of these neurons possessed numerous dendritic spines and that neurons receiving monosynaptic primary afferent input have a greater spine density than those receiving polysynaptic primary afferent input. These results reveal that intramedullary projection neurons represent a heterogeneous class of rNTS neurons and, through both intrinsic voltage-gated ion channels and local circuit interactions, transform incoming gustatory information into signals governing oromotor reflexive behaviors.
Keywords: patch clamp; primary afferent; taste.
Figures










References
-
- Bao H, Bradley RM, Mistretta CM. Development of intrinsic electrophysiological properties in neurons from the gustatory region of rat nucleus of solitary tract. Brain Res Dev Brain Res 86: 143–154, 1995 - PubMed
-
- Beckman ME, Whitehead MC. Intramedullary connections of the rostral nucleus of the solitary tract in the hamster. Brain Res 557: 265–279, 1991 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

