Competition driven by retinal waves promotes morphological and functional synaptic development of neurons in the superior colliculus
- PMID: 23741047
- PMCID: PMC3763158
- DOI: 10.1152/jn.01066.2012
Competition driven by retinal waves promotes morphological and functional synaptic development of neurons in the superior colliculus
Abstract
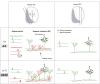
Prior to eye opening, waves of spontaneous activity sweep across the developing retina. These "retinal waves," together with genetically encoded molecular mechanisms, mediate the formation of visual maps in the brain. However, the specific role of wave activity in synapse development in retino-recipient brain regions is unclear. Here we compare the functional development of synapses and the morphological development of neurons in the superior colliculus (SC) of wild-type (WT) and transgenic (β2-TG) mice in which retinal wave propagation is spatially truncated (Xu HP, Furman M, Mineur YS, Chen H, King SL, Zenisek D, Zhou ZJ, Butts DA, Tian N, Picciotto MR, Crair MC. Neuron 70: 1115-1127, 2011). We use two recently developed brain slice preparations to examine neurons and synapses in the binocular vs. mainly monocular SC. We find that retinocollicular synaptic strength is reduced whereas the number of retinal inputs is increased in the binocular SC of β2-TG mice compared with WT mice. In contrast, in the mainly monocular SC the number of retinal inputs is normal in β2-TG mice, but, transiently, synapses are abnormally strong, possibly because of enhanced activity-dependent competition between local, "small" retinal wave domains. These findings demonstrate that retinal wave size plays an instructive role in the synaptic and morphological development of SC neurons, possibly through a competitive process among retinofugal axons.
Keywords: morphological development; nicotine; nicotinic acetylcholine receptor; retinal waves; superior colliculus; synapse maturation; visual map development.
Figures
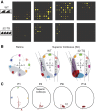
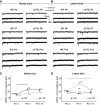

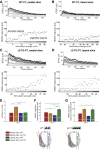
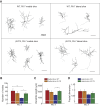
References
-
- Chen C, Regehr WG. Developmental remodeling of the retinogeniculate synapse. Neuron 28: 955–966, 2000 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

