The triterpenoid CDDO-Me inhibits bleomycin-induced lung inflammation and fibrosis
- PMID: 23741300
- PMCID: PMC3669327
- DOI: 10.1371/journal.pone.0063798
The triterpenoid CDDO-Me inhibits bleomycin-induced lung inflammation and fibrosis
Abstract
Pulmonary Fibrosis (PF) is a devastating progressive disease in which normal lung structure and function is compromised by scarring. Lung fibrosis can be caused by thoracic radiation, injury from chemotherapy and systemic diseases such as rheumatoid arthritis that involve inflammatory responses. CDDO-Me (Methyl 2-cyano-3,12-dioxooleana-1,9(11)dien-28-oate, Bardoxolone methyl) is a novel triterpenoid with anti-fibrotic and anti-inflammatory properties as shown by our in vitro studies. Based on this evidence, we hypothesized that CDDO-Me would reduce lung inflammation, fibrosis and lung function impairment in a bleomycin model of lung injury and fibrosis. To test this hypothesis, mice received bleomycin via oropharyngeal aspiration (OA) on day zero and CDDO-Me during the inflammatory phase from days -1 to 9 every other day. Bronchoalveolar lavage fluid (BALF) and lung tissue were harvested on day 7 to evaluate inflammation, while fibrosis and lung function were evaluated on day 21. On day 7, CDDO-Me reduced total BALF protein by 50%, alveolar macrophage infiltration by 40%, neutrophil infiltration by 90% (p≤0.01), inhibited production of the inflammatory cytokines KC and IL-6 by over 90% (p≤0.001), and excess production of the pro-fibrotic cytokine TGFβ by 50%. CDDO-Me also inhibited α-smooth muscle actin and fibronectin mRNA by 50% (p≤0.05). On day 21, CDDO-Me treatment reduced histological fibrosis, collagen deposition and αSMA production. Lung function was significantly improved at day 21 by treatment with CDDO-Me, as demonstrated by respiratory rate and dynamic compliance. These new findings reveal that CDDO-Me exhibits potent anti-fibrotic and anti-inflammatory properties in vivo. CDDO-Me is a potential new class of drugs to arrest inflammation and ameliorate fibrosis in patients who are predisposed to lung injury and fibrosis incited by cancer treatments (e.g. chemotherapy and radiation) and by systemic autoimmune diseases.
Conflict of interest statement
Figures


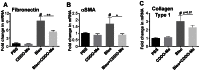




References
-
- Nathan SD, Noble PW, Tuder RM (2007) Idiopathic pulmonary fibrosis and pulmonary hypertension: connecting the dots. Am J Respir Crit Care Med 175: 875–880. - PubMed
-
- Camus P, Fanton A, Bonniaud P, Camus C, Foucher P (2004) Interstitial lung disease induced by drugs and radiation. Respiration 71: 301–326. - PubMed
-
- Limper AH (2004) Chemotherapy-induced lung disease. Clin Chest Med 25: 53–64. - PubMed
-
- Dimopoulou I, Bamias A, Lyberopoulos P, Dimopoulos MA (2006) Pulmonary toxicity from novel antineoplastic agents. Ann Oncol 17: 372–379. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 AI083206/AI/NIAID NIH HHS/United States
- T32HL066988/HL/NHLBI NIH HHS/United States
- R01 HL113495/HL/NHLBI NIH HHS/United States
- KL2RR024136/RR/NCRR NIH HHS/United States
- R01 EY017123/EY/NEI NIH HHS/United States
- R01 HL083761/HL/NHLBI NIH HHS/United States
- UL1 TR000042/TR/NCATS NIH HHS/United States
- T32ES007026/ES/NIEHS NIH HHS/United States
- R01 HL075432/HL/NHLBI NIH HHS/United States
- R01EY017123/EY/NEI NIH HHS/United States
- KL2 RR024136/RR/NCRR NIH HHS/United States
- T32 ES007026/ES/NIEHS NIH HHS/United States
- T32 HL066988/HL/NHLBI NIH HHS/United States
- R01HL075432/HL/NHLBI NIH HHS/United States
- 8UL1TR000042-07/TR/NCATS NIH HHS/United States
- R01HL083761/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

