Endolymphatic Na⁺ and K⁺ concentrations during cochlear growth and enlargement in mice lacking Slc26a4/pendrin
- PMID: 23741519
- PMCID: PMC3669272
- DOI: 10.1371/journal.pone.0065977
Endolymphatic Na⁺ and K⁺ concentrations during cochlear growth and enlargement in mice lacking Slc26a4/pendrin
Abstract
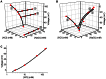
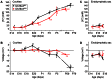
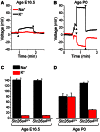
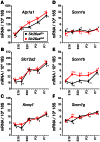
Slc26a4 (Δ/Δ) mice are deaf, develop an enlarged membranous labyrinth, and thereby largely resemble the human phenotype where mutations of SLC26A4 cause an enlarged vestibular aqueduct and sensorineural hearing loss. The enlargement is likely caused by abnormal ion and fluid transport during the time of embryonic development, however, neither the mechanisms of ion transport nor the ionic composition of the luminal fluid during this time of development are known. Here we determine the ionic composition of inner ear fluids at the time at which the enlargement develops and the onset of expression of selected ion transporters. Concentrations of Na(+) and K(+) were measured with double-barreled ion-selective electrodes in the cochlea and the endolymphatic sac of Slc26a4 (Δ/+), which develop normal hearing, and of Slc26a4 (Δ/Δ) mice, which fail to develop hearing. The expression of specific ion transporters was examined by quantitative RT-PCR and immunohistochemistry. High Na(+) (∼141 mM) and low K(+) concentrations (∼11 mM) were found at embryonic day (E) 16.5 in cochlear endolymph of Slc26a4 (Δ/+) and Slc26a4 (Δ/Δ) mice. Shortly before birth the K(+) concentration began to rise. Immediately after birth (postnatal day 0), the Na(+) and K(+) concentrations in cochlear endolymph were each ∼80 mM. In Slc26a4 (Δ/Δ) mice, the rise in the K(+) concentration occurred with a ∼3 day delay. K(+) concentrations were also found to be low (∼15 mM) in the embryonic endolymphatic sac. The onset of expression of the K(+) channel KCNQ1 and the Na(+)/2Cl(-)/K(+) cotransporter SLC12A2 occurred in the cochlea at E19.5 in Slc26a4 (Δ/+) and Slc26a4 (Δ/Δ) mice. These data demonstrate that endolymph, at the time at which the enlargement develops, is a Na(+)-rich fluid, which transitions into a K(+)-rich fluid before birth. The data suggest that the endolymphatic enlargement caused by a loss of Slc26a4 is a consequence of disrupted Na(+) transport.
Conflict of interest statement
Figures



References
-
- Albert S, Blons H, Jonard L, Feldmann D, Chauvin P, et al. (2006) SLC26A4 gene is frequently involved in nonsyndromic hearing impairment with enlarged vestibular aqueduct in Caucasian populations. Eur J Hum Genet 14: 773–779. - PubMed
-
- Anwar S, Riazuddin S, Ahmed ZM, Tasneem S, Ateeq uJ, et al. (2009) SLC26A4 mutation spectrum associated with DFNB4 deafness and Pendred's syndrome in Pakistanis. J Hum Genet 54: 266–270. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

