The modulation of apoptosis by oncogenic viruses
- PMID: 23741982
- PMCID: PMC3691765
- DOI: 10.1186/1743-422X-10-182
The modulation of apoptosis by oncogenic viruses
Abstract
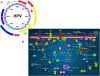
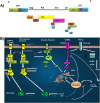
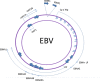
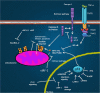
Transforming viruses can change a normal cell into a cancer cell during their normal life cycle. Persistent infections with these viruses have been recognized to cause some types of cancer. These viruses have been implicated in the modulation of various biological processes, such as proliferation, differentiation and apoptosis. The study of infections caused by oncogenic viruses had helped in our understanding of several mechanisms that regulate cell growth, as well as the molecular alterations leading to cancer. Therefore, transforming viruses provide models of study that have enabled the advances in cancer research. Viruses with transforming abilities, include different members of the Human Papillomavirus (HPV) family, Hepatitis C virus (HCV), Human T-cell Leukemia virus (HTLV-1), Epstein Barr virus (EBV) and Kaposi's Sarcoma Herpesvirus (KSHV).Apoptosis, or programmed cell death, is a tightly regulated process that plays an important role in development and homeostasis. Additionally, it functions as an antiviral defense mechanism. The deregulation of apoptosis has been implicated in the etiology of diverse diseases, including cancer. Oncogenic viruses employ different mechanisms to inhibit the apoptotic process, allowing the propagation of infected and damaged cells. During this process, some viral proteins are able to evade the immune system, while others can directly interact with the caspases involved in apoptotic signaling. In some instances, viral proteins can also promote apoptosis, which may be necessary for an accurate regulation of the initial stages of infection.
Figures


References
-
- The Nobel prizes. http://www.nobelprize.org.
-
- zur Hausen H. Viruses in human cancers. Science. 1991;254:1167–1173. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

