Intranasal immunization with a helper-dependent adenoviral vector expressing the codon-optimized fusion glycoprotein of human respiratory syncytial virus elicits protective immunity in BALB/c mice
- PMID: 23742026
- PMCID: PMC3685604
- DOI: 10.1186/1743-422X-10-183
Intranasal immunization with a helper-dependent adenoviral vector expressing the codon-optimized fusion glycoprotein of human respiratory syncytial virus elicits protective immunity in BALB/c mice
Abstract
Background: Human respiratory syncytial virus (RSV) is a serious pediatric pathogen of the lower respiratory tract. Currently, there is no clinically approved vaccine against RSV infection. Recent studies have shown that helper-dependent adenoviral (HDAd) vectors may represent effective and safe vaccine vectors. However, viral challenge has not been investigated following mucosal vaccination with HDAd vector vaccines.
Methods: To explore the role played by HDAd as an intranasally administered RSV vaccine vector, we constructed a HDAd vector encoding the codon optimized fusion glycoprotein (Fsyn) of RSV, designated HDAd-Fsyn, and delivered intranasally HDAd-Fsyn to mice.
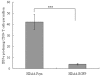
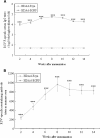
Results: RSV-specific humoral and cellular immune responses were generated in BALB/c mice, and serum IgG with neutralizing activity was significantly elevated after a homologous boost with intranasal (i.n.) application of HDAd-Fsyn. Humoral immune responses could be measured even 14 weeks after a single immunization. Immunization with i.n. HDAd-Fsyn led to effective protection against RSV infection on challenge.
Conclusion: The results indicate that HDAd-Fsyn can induce powerful systemic immunity against subsequent i.n. RSV challenge in a mouse model and is a promising candidate vaccine against RSV infection.
Figures


References
-
- Collins PL, Crowe JE. In: Fields virology. 5. Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editor. 2007. Respiratory syncytial virus and metapneumovirus; pp. 1601–1646.
-
- Karron RA. In: Vaccines. 5. Plotkin SA, Orenstein WA, Offit P, editor. Philadelphia: Saunders, Elsevier; 2008. Respiratory syncytial virus and parainfluenza virus vaccines; pp. 1283–1293.
-
- Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, Polack FP, Randolph VB, Deatly A, Hackell J. et al.Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. J Infect Dis. 2005;191:1093–1104. doi: 10.1086/427813. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

