Methodologies for the quantitative estimation of toxicant dose to cigarette smokers using physical, chemical and bioanalytical data
- PMID: 23742081
- PMCID: PMC3696342
- DOI: 10.3109/08958378.2013.794177
Methodologies for the quantitative estimation of toxicant dose to cigarette smokers using physical, chemical and bioanalytical data
Abstract
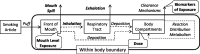
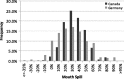
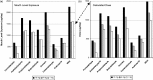
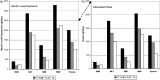
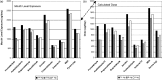
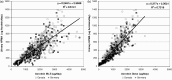
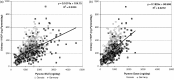
Methodologies have been developed, described and demonstrated that convert mouth exposure estimates of cigarette smoke constituents to dose by accounting for smoke spilled from the mouth prior to inhalation (mouth-spill (MS)) and the respiratory retention (RR) during the inhalation cycle. The methodologies are applicable to just about any chemical compound in cigarette smoke that can be measured analytically and can be used with ambulatory population studies. Conversion of exposure to dose improves the relevancy for risk assessment paradigms. Except for urinary nicotine plus metabolites, biomarkers generally do not provide quantitative exposure or dose estimates. In addition, many smoke constituents have no reliable biomarkers. We describe methods to estimate the RR of chemical compounds in smoke based on their vapor pressure (VP) and to estimate the MS for a given subject. Data from two clinical studies were used to demonstrate dose estimation for 13 compounds, of which only 3 have urinary biomarkers. Compounds with VP > 10(-5) Pa generally have RRs of 88% or greater, which do not vary appreciably with inhalation volume (IV). Compounds with VP < 10(-7) Pa generally have RRs dependent on IV and lung exposure time. For MS, mean subject values from both studies were slightly greater than 30%. For constituents with urinary biomarkers, correlations with the calculated dose were significantly improved over correlations with mouth exposure. Of toxicological importance is that the dose correlations provide an estimate of the metabolic conversion of a constituent to its respective biomarker.
Figures



References
-
- Apiezon M&I Materials Ltd; Manchester, UK: 2011. Apiezon high vacuum grease properties.http://www.apiezon.com/apiezon-vacuum-greases-properties-table.htm Available from: [Last accessed: 4 Dec 2011]
-
- Armitage AK, Dixon M, Frost BE, et al. The effect of tobacco blend additives on the retention of nicotine and solanesol in the human respiratory tract and on subsequent plasma nicotine concentrations during cigarette smoking. Chem Res Toxicol. 2004a;17:537–44. - PubMed
-
- Armitage AK, Dixon M, Frost BE, et al. The effect of inhalation volume and breath-hold duration on the retention of nicotine and solanesol in the human respiratory tract and on subsequent plasma nicotine concentrations during cigarette smoking. Beitr Tabakforsch Int. 2004b;21:240–9. - PubMed
-
- ATSDR Atlanta, GA: Agency for Toxic Substances and Disease Registry. U.S. Department of Health and Human Services; 1995. Toxicological profile for polycyclic aromatic hydrocarbons.http://www.atsdr.cdc.gov/toxprofiles/tp69.pdf Available from: [Last accessed: 1 Dec 2011] - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
