Acanthamoeba castellanii of the T4 genotype is a potential environmental host for Enterobacter aerogenes and Aeromonas hydrophila
- PMID: 23742105
- PMCID: PMC3682894
- DOI: 10.1186/1756-3305-6-169
Acanthamoeba castellanii of the T4 genotype is a potential environmental host for Enterobacter aerogenes and Aeromonas hydrophila
Abstract
Background: Acanthamoeba can interact with a wide range of microorganisms such as viruses, algae, yeasts, protists and bacteria including Legionella pneumophila, Pseudomonas aeruginosa, Vibrio cholerae, Helicobacter pylori, Listeria monocytogenes, Mycobacterium spp., and Escherichia coli. In this capacity, Acanthamoeba has been suggested as a vector in the transmission of bacterial pathogens to the susceptible hosts.
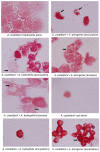
Methods: Here, we used a keratitis isolate of A. castellanii of the T4 genotype and studied its interactions with two bacterial genera which have not been tested before, Enterobacter aerogenes, and Aeromonas hydrophila, as well as E. coli. Assays were performed to determine bacterial association with and invasion of A. castellanii. Additionally, bacterial survival intracellular of A. castellanii trophozoites as well as cysts was determined.
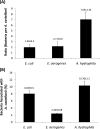
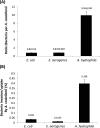
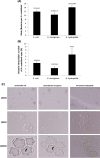
Results: All three bacterial isolates tested, associated, invaded, and survived inside A. castellanii trophozoites as well as A. castellanii cysts. However, E. aerogenes and E. coli exhibited significantly reduced association with and invasion of A. castellanii as compared with A. hydrophila (P < 0.01 using paired T-test, one tail distribution). In the long term survival assays, all three bacterial isolates tested remained viable inside A. castellanii trophozoites, while amoeba remained intact; however A. hydrophila exhibited higher survival inside amoebae (14.54 ± 3.3 bacteria:amoeba ratio) compared with E. aerogenes (3.96 ± 0.7 bacteria:amoeba ratio) and E. coli (5.85 ± 1.1 bacteria:amoeba ratio). A. hydrophila, E. coli, and E. aerogenes remained viable during the encystment process and exhibited higher levels of recovery from mature cysts (14.13 ± 0.89 A. hydrophila:amoeba ratio, 10.13 ± 1.17 E. aerogenes:amoeba ratio, and 11.95 ± 0.7 E. coli:amoeba ratio).
Conclusions: A. hydrophila and E. aerogenes also joined the ranks of other bacteria that could benefit from A. castellanii. Because cysts can be airborne, these findings suggest that Acanthamoeba is a potential vector in the transmission of A. hydrophila and E. aerogenes to susceptible hosts.
Figures

Similar articles
-
Interaction of Escherichia coli K1 and K5 with Acanthamoeba castellanii trophozoites and cysts.Korean J Parasitol. 2011 Dec;49(4):349-56. doi: 10.3347/kjp.2011.49.4.349. Epub 2011 Dec 16. Korean J Parasitol. 2011. PMID: 22355201 Free PMC article.
-
Impact of non-Legionella bacteria on the uptake and intracellular replication of Legionella pneumophila in Acanthamoeba castellanii and Naegleria lovaniensis.Microb Ecol. 2005 Nov;50(4):536-49. doi: 10.1007/s00248-005-0258-0. Epub 2005 Dec 15. Microb Ecol. 2005. PMID: 16341636
-
Escherichia coli interactions with Acanthamoeba: a symbiosis with environmental and clinical implications.J Med Microbiol. 2006 Jun;55(Pt 6):689-694. doi: 10.1099/jmm.0.46497-0. J Med Microbiol. 2006. PMID: 16687585
-
Encystment and Excystment Processes in Acanthamoeba castellanii: An Emphasis on Cellulose Involvement.Pathogens. 2025 Mar 10;14(3):268. doi: 10.3390/pathogens14030268. Pathogens. 2025. PMID: 40137753 Free PMC article. Review.
-
Encystment in Acanthamoeba castellanii: a review.Exp Parasitol. 2014 Nov;145 Suppl:S20-7. doi: 10.1016/j.exppara.2014.03.026. Epub 2014 Apr 12. Exp Parasitol. 2014. PMID: 24726698 Review.
Cited by
-
Constraint and opportunity in genome innovation.RNA Biol. 2014;11(3):186-96. doi: 10.4161/rna.27506. Epub 2013 Dec 20. RNA Biol. 2014. PMID: 24572460 Free PMC article. Review.
-
'Candidatus Cochliophilus cryoturris' (Coxiellaceae), a symbiont of the testate amoeba Cochliopodium minus.Sci Rep. 2017 Jun 13;7(1):3394. doi: 10.1038/s41598-017-03642-8. Sci Rep. 2017. PMID: 28611430 Free PMC article.
-
Protozoan Cysts Act as a Survival Niche and Protective Shelter for Foodborne Pathogenic Bacteria.Appl Environ Microbiol. 2015 Aug 15;81(16):5604-12. doi: 10.1128/AEM.01031-15. Epub 2015 Jun 12. Appl Environ Microbiol. 2015. PMID: 26070667 Free PMC article.
-
Increased Sensitivity of Amoeba-Grown Francisella Species to Disinfectants.Microorganisms. 2020 Aug 20;8(9):1260. doi: 10.3390/microorganisms8091260. Microorganisms. 2020. PMID: 32825290 Free PMC article.
-
Variables Affecting the Recovery of Acanthamoeba Trophozoites.Pathogens. 2021 Feb 18;10(2):221. doi: 10.3390/pathogens10020221. Pathogens. 2021. PMID: 33670669 Free PMC article.
References
-
- Castellani A. An amoeba found in culture of yeast: preliminary note. J Trop Med Hyg. 1930;33:160.
-
- Douglas M. Notes on the classification of the amoeba found by Castellani on culture of a yeast-like fungus. J Trop Med Hyg. 1930;33:258–59.
-
- Volkonsky M. Hartmanella castellanii Douglas, et classification des hartmannelles. Arch Zool Exp Gen. 1931;72:317–39.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

