Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis. A pilot study using inhaled hypertonic saline
- PMID: 23742699
- PMCID: PMC3778738
- DOI: 10.1164/rccm.201302-0219OC
Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis. A pilot study using inhaled hypertonic saline
Abstract
Rationale: Lung clearance index (LCI), measured by multiple breath washout (MBW), is a noninvasive measure of ventilation inhomogeneity that holds promise as an objective physiologic endpoint for clinical trials in infants and preschool children with cystic fibrosis (CF).
Objectives: To study the feasibility of using LCI to assess treatment effect outcomes in CF trials of infants and preschoolers.
Methods: The Infant Study of Inhaled Saline trial was a multicenter, randomized, controlled trial of hypertonic (7%) versus isotonic (0.9%) saline inhaled twice daily for 48 weeks in children with CF under 6 years of age. LCI measurements were performed in a single-center pilot substudy at baseline and 48 weeks using a respiratory mass spectrometer and sulfur hexafluoride as the tracer gas. LCI measurements were standardized using published normative data (zLCI) to account for height-related changes in LCI during early childhood. A generalized estimating equation model with an interaction between treatment group and test occasion was used to estimate a treatment effect.
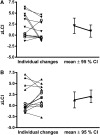
Measurements and main results: A total of 27 participants were randomized; 25 participants, aged (median [range]) 2.6 (0.34-4.95) years, had acceptable baseline and follow-up LCI measures. On average, LCI decreased in the hypertonic saline group (n = 12) by 1.19 z-scores units (95% confidence interval [CI] = -2.46 to 0.06), and remained stable in the isotonic saline group (n = 13) at 0.81 (95% CI = -0.40 to 2.02). A significant treatment effect was observed for zLCI (2.01; 95% CI = 0.26 to 3.76; P = 0.025).
Conclusions: MBW testing is feasible in an interventional study in infants and preschool children with CF. These pilot findings support the development of MBW and LCI as an objective outcome measure in interventional trials in young children with CF, and provide estimates for sample size calculations for future studies.
Trial registration: ClinicalTrials.gov NCT00709280.
Figures

Comment in
-
Early intervention for newborns screened for cystic fibrosis.Am J Respir Crit Care Med. 2013 Aug 15;188(4):409-10. doi: 10.1164/rccm.201306-1023ED. Am J Respir Crit Care Med. 2013. PMID: 23947515 No abstract available.
References
-
- Cantin A. Cystic fibrosis lung inflammation: early, sustained, and severe. Am J Respir Crit Care Med. 1995;151:939–941. - PubMed
-
- Long FR, Williams RS, Castile RG. Structural airway abnormalities in infants and young children with cystic fibrosis. J Pediatr. 2004;144:154–161. - PubMed
-
- Mott LS, Park J, Murray CP, Gangell CL, de Klerk NH, Robinson PJ, Robertson CF, Ranganathan SC, Sly PD, Stick SM AREST CF. Progression of early structural lung disease in young children with cystic fibrosis assessed using CT. Thorax. 2012;67:509–516. - PubMed
-
- Gustafsson PM, Aurora P, Lindblad A. Evaluation of ventilation maldistribution as an early indicator of lung disease in children with cystic fibrosis. Eur Respir J. 2003;22:972–979. - PubMed
-
- Aurora P, Stanojevic S, Wade A, Oliver C, Kozlowska W, Lum S, Bush A, Price J, Carr SB, Shankar A, et al. London Cystic Fibrosis Collaboration. Lung clearance index at 4 years predicts subsequent lung function in children with cystic fibrosis. Am J Respir Crit Care Med. 2011;183:752–758. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

