Neuromodulatory control of sleep in Drosophila melanogaster: integration of competing and complementary behaviors
- PMID: 23743247
- PMCID: PMC3783581
- DOI: 10.1016/j.conb.2013.05.003
Neuromodulatory control of sleep in Drosophila melanogaster: integration of competing and complementary behaviors
Abstract
The transition between wake and sleep states is characterized by rapid and generalized changes in both sensory and motor processing. Sleep is antagonistic to the expression of important behaviors, like feeding, reproduction and learning whose relative importance to an individual will depend on its circumstances at that moment. An understanding of how the decision to sleep is affected by these other drives and how this process is coordinated across the entire brain remains elusive. Neuromodulation is an important regulatory feature of many behavioral circuits and the reconfiguring of these circuits by modulators can have both long-term and short-term consequences. Drosophila melanogaster has become an important model system for understanding the molecular and genetic bases of behaviors and in recent years neuromodulatory systems have been shown to play a major role in regulation of sleep and other behaviors in this organism. The fly, with its increasingly well-defined behavioral circuitry and powerful genetic tools, is a system poised to provide new insight into the complex issue of how neuromodulation can coordinate situation-specific behavioral needs with the brain's arousal state.
Copyright © 2013 Elsevier Ltd. All rights reserved.
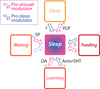
Figures

References
-
- Rechtschaffen A, Gilliland MA, Bergmann BM, Winter JB. Physiological correlates of prolonged sleep deprivation in rats. Science. 1983;221:182–184. - PubMed
-
- Shaw PJ, Tononi G, Greenspan RJ, Robinson DF. Stress response genes protect against lethal effects of sleep deprivation in Drosophila. Nature. 2002;417:287–291. - PubMed
-
- Lugaresi E, Provini F, Cortelli P. Agrypnia excitata. Sleep Med. 2011;12(Suppl 2):S3–S10. - PubMed
-
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. - PubMed
-
-
Various. Reviews on neuromodulatory mechanisms. Neuron. 2012;76:1–256. This special issue on neuromodulation covers a lot of ground, from molecular mechanisms to computational treatments of modulation.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

