Is it necessary to use three mandatory loading doses when commencing therapy for neovascular age-related macular degeneration using bevacizumab? (BeMOc Trial)
- PMID: 23743535
- PMCID: PMC3740324
- DOI: 10.1038/eye.2013.93
Is it necessary to use three mandatory loading doses when commencing therapy for neovascular age-related macular degeneration using bevacizumab? (BeMOc Trial)
Abstract
Purpose: To determine whether a Pro Re Nata (PRN) regimen with three initial mandatory loading doses results in better functional and anatomical outcome compared with a PRN regimen without initial loading when using intravitreal bevacizumab in patients with minimal classic or occult choroidal neovascularisation secondary to age-related macular degeneration.
Methods: Patients were randomised (1 : 1) to Loading (LD group) or No Loading (NLD group) and treated with open label intravitreal bevacizumab. In the LD group, patients received two mandatory doses after the baseline dose before entering the PRN phase and in the NLD group, patients did not receive mandatory doses after the baseline dose. Six-weekly evaluations were performed up to week 54 and retreatment was done based on OCT criteria. Visual stability and reduction in central retinal thickness were compared between groups.
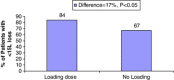
Results: 49 patients were in the NLD group and 50 patients were in the LD group. At the 12-month end point, 84% of the patients in the LD group achieved visual stability (<15 letter loss) compared with 67% of the patients in the NLD group (P<0.05). The mean reduction in central macular thickness was 105.35 μm in the LD group and 81.45 μm in the NLD group (P>0.05). There was no significant difference in scores of VFQ-25 questionnaire testing between the two groups and no serious ocular or systemic side effects were observed.
Conclusion: The results supported our hypothesis that a loading dose leads to slightly better visual stability in terms of proportions of patients experiencing moderate visual loss, but did not support the hypothesised difference in anatomical outcome.
Figures
References
-
- Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, Wordsworth S, et al. Ranibizumab versus bevacizumab to treat neovascular age-related macular degeneration: one-year findings from the IVAN randomized trial. Ophthalmology. 2012;119:1399–1411. - PubMed
-
- Arias L, Caminal JM, Casas L, Masuet C, Badia MB, Rubio M, et al. A study comparing two protocols of treatment with intravitreal bevacizumab (Avastin) for neovascular age-related macular degeneration. Br J Ophthalmol. 2008;92:1636–1641. - PubMed
-
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Comparison of age-related macular degeneration treatments trials (catt) research group, ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two year results. Ophthalmology. 2012;119:1388–1398. - PubMed
-
- Gupta B, Adewoyin T, Patel SK, Sivaprasad S. Comparison of two intravitreal ranibizumab treatment schedules for neovascular age-related macular degeneration. Br J Ophthalmol. 2011;95 (3:386–390. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials