Parents' interest in whole-genome sequencing of newborns
- PMID: 23743552
- PMCID: PMC4164384
- DOI: 10.1038/gim.2013.76
Parents' interest in whole-genome sequencing of newborns
Abstract
Purpose: The aim of this study was to assess parents' interest in whole-genome sequencing for newborns.
Methods: We conducted a survey of a nationally representative sample of 1,539 parents about their interest in whole-genome sequencing of newborns. Participants were randomly presented with one of two scenarios that differed in the venue of testing: one offered whole-genome sequencing through a state newborn screening program, whereas the other offered whole-genome sequencing in a pediatrician's office.
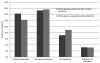
Results: Overall interest in having future newborns undergo whole-genome sequencing was generally high among parents. If whole-genome sequencing were offered through a state's newborn-screening program, 74% of parents were either definitely or somewhat interested in utilizing this technology. If offered in a pediatrician's office, 70% of parents were either definitely or somewhat interested. Parents in both groups most frequently identified test accuracy and the ability to prevent a child from developing a disease as "very important" in making a decision to have a newborn's whole genome sequenced.
Conclusion: These data may help health departments and children's health-care providers anticipate parents' level of interest in genomic screening for newborns. As whole-genome sequencing is integrated into clinical and public health services, these findings may inform the development of educational strategies and outreach messages for parents.
Conflict of interest statement
Disclosure: The authors declare no conflict of interest.
Figures
References
-
- National Newborn Screening and Genetics Resource Center. [Accessed 3 December 2012];National newborn screening report. http://genes-rus.uthscsa.edu/resources/newborn/00/ch2_ complete.pdf.
-
- Paul DB. Task Force on Genetic Testing (U.S.) The history of newborn phenylketonuria screening in the U.S. In: Holtzman NA, Watson MS, editors. Promoting Safe and Effective Genetic Testing in the United States: Final Report of the Task Force on Genetic Testing. xxiv. Johns Hopkins University Press; Baltimore, MD: 1998. p. 186.
-
- Secretary's Advisory Committee on Heritable Disorders in Newborns and Children. Recommended Uniform Screening Panel of the Secretary's Advisory Committee on Heritable Disorders in Newborns and Children. [Accessed 7 December 2012]; http://www.hrsa.gov/advisorycommittees/mchbadvisory/heritabledisorders/r....
-
- Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. 2003;49:1797–1817. - PubMed
-
- Comeau AM, Parad RB, Dorkin HL, et al. Population-based newborn screening for genetic disorders when multiple mutation DNA testing is incorporated: a cystic fibrosis newborn screening model demonstrating increased sensitivity but more carrier detections. Pediatrics. 2004;113:1573–1581. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials