Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements
- PMID: 23743568
- PMCID: PMC3740724
- DOI: 10.1158/1078-0432.CCR-13-0895
Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements
Abstract
Purpose: Immune-related response criteria (irRC) was developed to adequately assess tumor response to immunotherapy. The irRC are based on bidimensional measurements, as opposed to unidimensional measurements defined by Response Evaluation Criteria in Solid Tumors, which has been widely used in solid tumors. We aimed to compare response assessment by bidimensional versus unidimensional irRC in patients with advanced melanoma treated with ipilimumab.
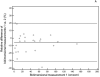
Experimental design: Fifty-seven patients with advanced melanoma treated with ipilimumab in a phase II, expanded access trial were studied. Bidimensional tumor measurement records prospectively conducted during the trial were reviewed to generate a second set of measurements using unidimensional, longest diameter measurements. The percent changes of measurements at follow-up, best overall response, and time-to-progression (TTP) were compared between bidimensional and unidimensional irRC. Interobserver variability for bidimensional and unidimensional measurements was assessed in 25 randomly selected patients.
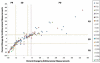
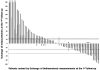
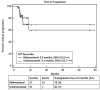
Results: The percent changes at follow-up scans were highly concordant between the 2 criteria (Spearman r: 0.953-0.965, first to fourth follow-up). The best immune-related response was highly concordant between the 2 criteria (κw = 0.881). TTP was similar between the bidimensional and unidimensional assessments (progression-free at 6 months: 70% vs. 81%, respectively). The unidimensional measurements were more reproducible than bidimensional measurements, with the 95% limits of agreement of (-16.1%, 5.8%) versus (-31.3%, 19.7%), respectively.
Conclusion: irRC using the unidimensional measurements provided highly concordant response assessment compared with the bidimensional irRC, with less measurement variability. The use of unidimensional irRC is proposed to assess response to immunotherapy in solid tumors, given its simplicity, higher reproducibility, and high concordance with the bidimensional irRC.
Figures

References
-
- Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, et al. A randomized, double-blind, placebo controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clin Cancer Res. 2009;15:5591–8. - PubMed
-
- Wolchok JD, Neyns B, Linette G, Negrier S, Lutzky J, Thomas L, et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 2010;11:155–64. - PubMed
-
- O'Day, Maio M, Ciarion-Sileni V, Gajewski TF, Pehamberger H, Bondarenko IN, et al. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: a multicenter single-arm phase II study. Ann Oncol. 2010 Aug;21(8):1712–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

