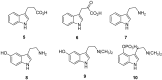
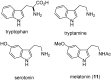
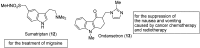
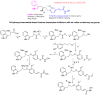
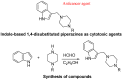
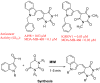
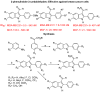
Biomedical importance of indoles
- PMID: 23743888
- PMCID: PMC6270133
- DOI: 10.3390/molecules18066620
Biomedical importance of indoles
Abstract
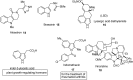
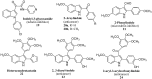
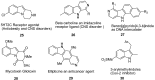
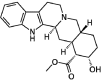
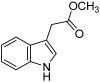
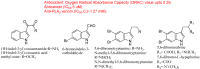
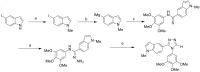
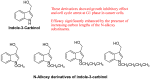
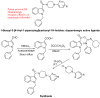
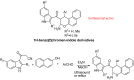
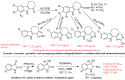
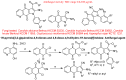
The indole nucleus is an important element of many natural and synthetic molecules with significant biological activity. This review covers some of the relevant and recent achievements in the biological, chemical and pharmacological activity of important indole derivatives in the areas of drug discovery and analysis.
Figures
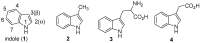

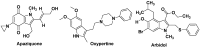
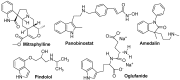
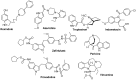
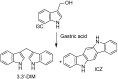
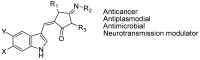
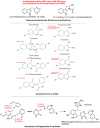

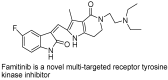
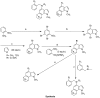
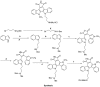


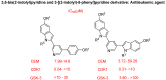



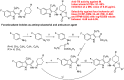
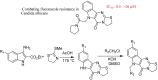
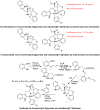

References
-
- Dolle R.E., Nelson K.H. Comprehensive survey of combinatorial library synthesis: 1998. J. Comb. Chem. 1999;1:235–282. - PubMed
-
- Hanessian S., McNaughton-Smith G., Lombart H.G., Lubell W.D. Design and synthesis of conformationally constrained amino acids as versatile scaffolds and peptide mimetics. Tetrahedron. 1997;53:12789–12854. doi: 10.1016/S0040-4020(97)00476-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

