Reduction potentials of heterometallic manganese-oxido cubane complexes modulated by redox-inactive metals
- PMID: 23744039
- PMCID: PMC3690856
- DOI: 10.1073/pnas.1302677110
Reduction potentials of heterometallic manganese-oxido cubane complexes modulated by redox-inactive metals
Abstract
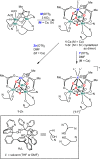
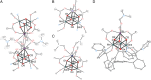
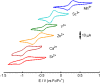
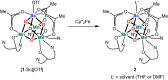
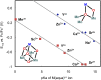
Understanding the effect of redox-inactive metals on the properties of biological and heterogeneous water oxidation catalysts is important both fundamentally and for improvement of future catalyst designs. In this work, heterometallic manganese-oxido cubane clusters [MMn3O4] (M = Sr(2+), Zn(2+), Sc(3+), Y(3+)) structurally relevant to the oxygen-evolving complex (OEC) of photosystem II were prepared and characterized. The reduction potentials of these clusters and other related mixed metal manganese-tetraoxido complexes are correlated with the Lewis acidity of the apical redox-inactive metal in a manner similar to a related series of heterometallic manganese-dioxido clusters. The redox potentials of the [SrMn3O4] and [CaMn3O4] clusters are close, which is consistent with the observation that the OEC is functional only with one of these two metals. Considering our previous studies of [MMn3O2] moieties, the present results with more structurally accurate models of the OEC ([MMn3O4]) suggest a general relationship between the reduction potentials of heterometallic oxido clusters and the Lewis acidities of incorporated cations that applies to diverse structural motifs. These findings support proposals that one function of calcium in the OEC is to modulate the reduction potential of the cluster to allow electron transfer.
Keywords: electrochemistry; heterometallic complexes; manganese clusters; model complexes; photosynthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
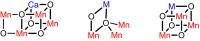
References
-
- Yocum CF. The calcium and chloride requirements of the O2 evolving complex. Coord Chem Rev. 2008;252(3-4):296–305.
-
- Ghanotakis DF, Babcock GT, Yocum CF. Calcium reconstitutes high rates of oxygen evolution in polypeptide depleted Photosystem II preparations. FEBS Lett. 1984;167(1):127–130.
-
- Vrettos JS, Stone DA, Brudvig GW. Quantifying the ion selectivity of the Ca2+ site in photosystem II: Evidence for direct involvement of Ca2+ in O2 formation. Biochemistry. 2001;40(26):7937–7945. - PubMed
-
- Lee C-I, Lakshmi KV, Brudvig GW. Probing the functional role of Ca2+ in the oxygen-evolving complex of photosystem II by metal ion inhibition. Biochemistry. 2007;46(11):3211–3223. - PubMed
-
- Pecoraro VL, Baldwin MJ, Caudle MT, Hsieh WY, Law NA. A proposal for water oxidation in photosystem II. Pure Appl Chem. 1998;70(4):925–929.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

