Altered cortical GABAA receptor composition, physiology, and endocytosis in a mouse model of a human genetic absence epilepsy syndrome
- PMID: 23744069
- PMCID: PMC3774412
- DOI: 10.1074/jbc.M112.444372
Altered cortical GABAA receptor composition, physiology, and endocytosis in a mouse model of a human genetic absence epilepsy syndrome
Abstract
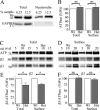
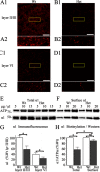
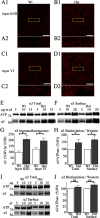
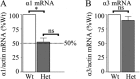
Patients with generalized epilepsy exhibit cerebral cortical disinhibition. Likewise, mutations in the inhibitory ligand-gated ion channels, GABAA receptors (GABAARs), cause generalized epilepsy syndromes in humans. Recently, we demonstrated that heterozygous knock-out (Hetα1KO) of the human epilepsy gene, the GABAAR α1 subunit, produced absence epilepsy in mice. Here, we determined the effects of Hetα1KO on the expression and physiology of GABAARs in the mouse cortex. We found that Hetα1KO caused modest reductions in the total and surface expression of the β2 subunit but did not alter β1 or β3 subunit expression, results consistent with a small reduction of GABAARs. Cortices partially compensated for Hetα1KO by increasing the fraction of residual α1 subunit on the cell surface and by increasing total and surface expression of α3, but not α2, subunits. Co-immunoprecipitation experiments revealed that Hetα1KO increased the fraction of α1 subunits, and decreased the fraction of α3 subunits, that associated in hybrid α1α3βγ receptors. Patch clamp electrophysiology studies showed that Hetα1KO layer VI cortical neurons exhibited reduced inhibitory postsynaptic current peak amplitudes, prolonged current rise and decay times, and altered responses to benzodiazepine agonists. Finally, application of inhibitors of dynamin-mediated endocytosis revealed that Hetα1KO reduced base-line GABAAR endocytosis, an effect that probably contributes to the observed changes in GABAAR expression. These findings demonstrate that Hetα1KO exerts two principle disinhibitory effects on cortical GABAAR-mediated inhibitory neurotransmission: 1) a modest reduction of GABAAR number and 2) a partial compensation with GABAAR isoforms that possess physiological properties different from those of the otherwise predominant α1βγ GABAARs.
Keywords: Brain; Confocal Microscopy; Electrophysiology; Endocytosis; Endoplasmic Reticulum (ER); Epilepsy; GABA Receptors; Glycosylation; Membrane Trafficking; Western Blotting.
Figures





References
-
- Shu Y., Hasenstaub A., McCormick D. A. (2003) Turning on and off recurrent balanced cortical activity. Nature 423, 288–293 - PubMed
-
- Lüttjohann A., Zhang S., de Peijper R., van Lijtelaar G. (2011) Electrical stimulation of the epileptic focus in absence epileptic WAG/Rij rats. Assessment of local and network excitability. Neuroscience 188, 125–134 - PubMed
-
- Badawy R. A., Jackson G. D. (2012) Cortical excitability in migraine and epilepsy. A common feature? J. Clin. Neurophysiol. 29, 244–249 - PubMed
-
- Fedi M., Berkovic S. F., Macdonell R. A., Curatolo J. M., Marini C., Reutens D. C. (2008) Intracortical hyperexcitability in humans with a GABAA receptor mutation. Cereb. Cortex 18, 664–669 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

