Carbamazepine as a novel small molecule corrector of trafficking-impaired ATP-sensitive potassium channels identified in congenital hyperinsulinism
- PMID: 23744072
- PMCID: PMC3774364
- DOI: 10.1074/jbc.M113.470948
Carbamazepine as a novel small molecule corrector of trafficking-impaired ATP-sensitive potassium channels identified in congenital hyperinsulinism
Abstract
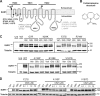
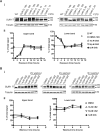
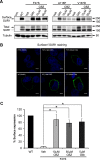
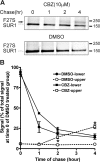
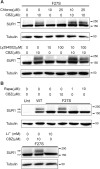
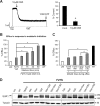
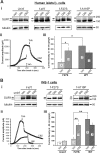
ATP-sensitive potassium (KATP) channels consisting of sulfonylurea receptor 1 (SUR1) and the potassium channel Kir6.2 play a key role in insulin secretion by coupling metabolic signals to β-cell membrane potential. Mutations in SUR1 and Kir6.2 that impair channel trafficking to the cell surface lead to loss of channel function and congenital hyperinsulinism. We report that carbamazepine, an anticonvulsant, corrects the trafficking defects of mutant KATP channels previously identified in congenital hyperinsulinism. Strikingly, of the 19 SUR1 mutations examined, only those located in the first transmembrane domain of SUR1 responded to the drug. We show that unlike that reported for several other protein misfolding diseases, carbamazepine did not correct KATP channel trafficking defects by activating autophagy; rather, it directly improved the biogenesis efficiency of mutant channels along the secretory pathway. In addition to its effect on channel trafficking, carbamazepine also inhibited KATP channel activity. Upon subsequent removal of carbamazepine, however, the function of rescued channels was recovered. Importantly, combination of the KATP channel opener diazoxide and carbamazepine led to enhanced mutant channel function without carbamazepine washout. The corrector effect of carbamazepine on mutant KATP channels was also demonstrated in rat and human β-cells with an accompanying increase in channel activity. Our findings identify carbamazepine as a novel small molecule corrector that may be used to restore KATP channel expression and function in a subset of congenital hyperinsulinism patients.
Keywords: ABC Transporter; CFTR; Carbamazepine; Hyperinsulinism; Intracellular Trafficking; KATP Channel; Molecular Chaperone; Potassium Channels; Sulfonylurea Receptor 1; β-Cells.
Figures
References
-
- Stanley C. A. (2002) Advances in diagnosis and treatment of hyperinsulinism in infants and children. J. Clin. Endocrinol. Metab. 87, 4857–4859 - PubMed
-
- Dunne M. J., Cosgrove K. E., Shepherd R. M., Aynsley-Green A., Lindley K. J. (2004) Hyperinsulinism in infancy: from basic science to clinical disease. Physiol. Rev. 84, 239–275 - PubMed
-
- Glaser B., Thornton P. S., Herold K., Stanley C. A. (1998) Clinical and molecular heterogeneity of familial hyperinsulinism. J. Pediatr. 133, 801–802 - PubMed
-
- Gloyn A. L., Siddiqui J., Ellard S. (2006) Mutations in the genes encoding the pancreatic β-cell KATP channel subunits Kir6.2 (KCNJ11) and SUR1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum. Mutat. 27, 220–231 - PubMed
-
- Aguilar-Bryan L., Bryan J. (1999) Molecular biology of adenosine triphosphate-sensitive potassium channels. Endocr. Rev. 20, 101–135 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

