A novel Osmium-based compound targets the mitochondria and triggers ROS-dependent apoptosis in colon carcinoma
- PMID: 23744353
- PMCID: PMC3698552
- DOI: 10.1038/cddis.2013.185
A novel Osmium-based compound targets the mitochondria and triggers ROS-dependent apoptosis in colon carcinoma
Abstract
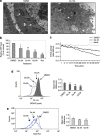
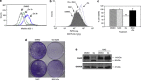
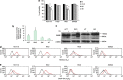
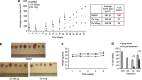
Engagement of the mitochondrial-death amplification pathway is an essential component in chemotherapeutic execution of cancer cells. Therefore, identification of mitochondria-targeting agents has become an attractive avenue for novel drug discovery. Here, we report the anticancer activity of a novel Osmium-based organometallic compound (hereafter named Os) on different colorectal carcinoma cell lines. HCT116 cell line was highly sensitive to Os and displayed characteristic features of autophagy and apoptosis; however, inhibition of autophagy did not rescue cell death unlike the pan-caspase inhibitor z-VAD-fmk. Furthermore, Os significantly altered mitochondrial morphology, disrupted electron transport flux, decreased mitochondrial transmembrane potential and ATP levels, and triggered a significant increase in reactive oxygen species (ROS) production. Interestingly, the sensitivity of cell lines to Os was linked to its ability to induce mitochondrial ROS production (HCT116 and RKO) as HT29 and SW620 cell lines that failed to show an increase in ROS were resistant to the death-inducing activity of Os. Finally, intra-peritoneal injections of Os significantly inhibited tumor formation in a murine model of HCT116 carcinogenesis, and pretreatment with Os significantly enhanced tumor cell sensitivity to cisplatin and doxorubicin. These data highlight the mitochondria-targeting activity of this novel compound with potent anticancer effect in vitro and in vivo, which could have potential implications for strategic therapeutic drug design.
Figures


References
-
- Tan ML, Ooi JP, Ismail N, Moad AI, Muhammad TS. Programmed cell death pathways and current antitumor targets. Pharm Res. 2009;26:1547–1560. - PubMed
-
- Dias N, Bailly C. Drugs targeting mitochondrial functions to control tumor cell growth. Biochem Pharmacol. 2005;70:1–12. - PubMed
-
- Fulda S, Galluzzi L, Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. - PubMed
-
- Modica-Napolitano JS, Kulawiec M, Singh KK. Mitochondria and human cancer. Curr Mol Med. 2007;7:121–131. - PubMed
-
- Neuzil J, Dong LF, Rohlena J, Truksa J, Ralph SJ. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion. 2012;13:199–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

