Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia
- PMID: 23744449
- PMCID: PMC7064814
- DOI: 10.1002/14651858.CD001444.pub2
Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia
Abstract
Background: Iron deficiency and iron deficiency anaemia (IDA) are common in young children. It has been suggested that the lack of iron may have deleterious effects on children's psychomotor development and cognitive function. To evaluate the benefits of iron therapy on psychomotor development and cognitive function in children with IDA, a Cochrane review was carried out in 2001. This is an update of that review.
Objectives: To determine the effects of iron therapy on psychomotor development and cognitive function in iron deficient anaemic children less than three years of age.
Search methods: We searched the following databases in April 2013: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, PsycINFO, LILACS, ClinicalTrials.gov and World Health Organization International Clinical Trials Registry Platform (ICTRP). We also searched the reference lists of review articles and reports, and ran citation searches in the Science Citation Index for relevant studies identified by the primary search. We also contacted key authors.
Selection criteria: Studies were included if children less than three years of age with evidence of IDA were randomly allocated to iron or iron plus vitamin C versus a placebo or vitamin C alone, and assessment of developmental status or cognitive function was carried out using standardised tests by observers blind to treatment allocation.
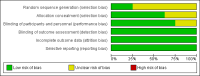
Data collection and analysis: Two review authors independently screened titles and abstracts retrieved from the searches and assessed full-text copies of all potentially relevant studies against the inclusion criteria. The same review authors independently extracted data and assessed the risk of bias of the eligible studies. Data were analysed separately depending on whether assessments were performed within one month of beginning iron therapy or after one month.
Main results: We identified one eligible study in the update search that had not been included in the original review. In total, we included eight trials.Six trials, including 225 children with IDA, examined the effects of iron therapy on measures of psychomotor development and cognitive function within 30 days of commencement of therapy. We could pool data from five trials. The pooled difference in pre- to post-treatment change in Bayley Scale Psychomotor Development Index (PDI) between iron and placebo groups was -1.25 (95% confidence interval (CI) -4.56 to 2.06, P value = 0.65; I(2) = 33% for heterogeneity, random-effects meta-analysis; low quality evidence) and in Bayley Scale Mental Development Index (MDI) was 1.04 (95% CI -1.30 to 3.39, P value = 0.79; I(2) = 31% for heterogeneity, random-effects meta-analysis; low quality evidence).Two studies, including 160 randomised children with IDA, examined the effects of iron therapy on measures of psychomotor development and cognitive function more than 30 days after commencement of therapy. One of the studies reported the mean number of skills gained after two months of iron therapy using the Denver Developmental Screening Test. The intervention group gained 0.8 (95% CI -0.18 to 1.78, P value = 0.11, moderate quality of evidence) more skills on average than the control group. The other study reported that the difference in pre- to post-treatment change in Bayley Scale PDI between iron-treated and placebo groups after four months was 18.40 (95% CI 10.16 to 26.64, P value < 0.0001; moderate quality evidence) and in Bayley Scale MDI was 18.80 (95% CI 10.17 to 27.43, P value < 0.0001; moderate quality evidence).
Authors' conclusions: There is no convincing evidence that iron treatment of young children with IDA has an effect on psychomotor development or cognitive function within 30 days after commencement of therapy. The effect of longer-term treatment remains unclear. There is an urgent need for further large randomised controlled trials with long-term follow-up.
Conflict of interest statement
Bo Wang ‐ none known. Si‐Yan Zhan ‐ none known. Ting Gong ‐ none known. Liming Lee ‐ none known.
Figures


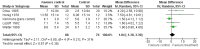










Update of
-
Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia.Cochrane Database Syst Rev. 2001;(2):CD001444. doi: 10.1002/14651858.CD001444. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2013 Jun 06;(6):CD001444. doi: 10.1002/14651858.CD001444.pub2. PMID: 11405989 Updated.
References
References to studies included in this review
Aukett 1986 {published data only}
Driva 1985 {published data only}
-
- Driva A, Kafatos A, Solman M. Iron deficiency and the cognitive and psychomotor development of children: a pilot study with institutionalised children. Early Child Development and Care 1985;22(1):73‐82.
Honig 1978 {published data only}
Idjradinata 1993 {published data only}
-
- Idjradinata P, Pollitt E. Reversal of developmental delays in iron‐deficient anaemic infants treated with iron. Lancet 1993;341(8836):1‐4. - PubMed
Kimmons [pers comm] {unpublished data only}
-
- Kimmons G, Moffatt MEK, Lonstaffe S, Whalen‐Besant J (University of Manitoba, Winnipeg, Canada). Short term effects of intramuscular iron on the behaviour of iron‐deficient children: a clinical trial [personal communication]. Letter to: Susana Martins (Federal University of São Paulo, São Paulo, Brazil) 30 November 2000.
Lozoff 1982 {published data only}
-
- Lozoff B, Brittenham GM, Viteri FE, Abraham WW, Urrutia JJ. The effects of short‐term oral iron therapy on developmental deficits in iron‐deficient anemic infants. Journal of Pediatrics 1982;100(3):351‐7. - PubMed
Lozoff 1987 {published data only}
-
- Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez R, et al. Iron deficiency anemia and iron therapy effects on infant developmental test performance. Pediatrics 1987;79(6):981‐95. - PubMed
Walter 1989 {published data only}
References to studies excluded from this review
Deinard 1986 {published data only}
-
- Deinard AS, List A, Lindgren B, Hunt JV, Chang PN. Cognitive deficits in iron‐deficient and iron deficient anemic children. Journal of Pediatrics 1986;108(5):681‐9. - PubMed
Linos 1990 {published data only}
-
- Gonzalez de Aledo Linos A, Rollán Rollán A, Bonilla Miera C. Prospective study of the prevalence of iron deficiency in breast fed infants in Cantabria, its relation to the introduction of cows milk and psychomotor development [Estudio prospectivo sobre la prevalencia de ferropenia en lactantes de Cantabria, su relacion con la introduccion de la leche de vaca el desarrollo psicomotor]. Anales Españoles de Pediatría 1990;32(1):24‐7. - PubMed
Lozoff 1996 {published data only}
-
- Lozoff B, Wolf AW, Jimenez E. Iron deficiency anemia and infant development: effects of extended oral iron therapy. Journal of Pediatrics 1996;129(3):382‐9. - PubMed
Lozoff 2003 {published data only}
-
- Lozoff B, Andraca I, Castillo M, Smith JB, Walter T, Pino P. Behavioral and developmental effects of preventing iron‐deficiency anemia in healthy full‐term infants. Pediatrics 2003;112(4):846‐54. - PubMed
Moffatt 1994 {published data only}
-
- Moffatt MEK, Longstaffe S, Besant J, Dureski C. Prevention of iron deficiency and psychomotor decline in high risk infants through use of iron fortified infant formula: a randomised clinical trial. Journal of Pediatrics 1994;125(4):527–34. - PubMed
Oski 1983 {published data only}
-
- Oski FA, Honig AS, Helu B, Howanitz P. Effects of iron therapy on behaviour performance in nonanemic, iron‐deficient infants. Pediatrics 1983;71(6):877‐80. - PubMed
Stoltzfus 2001 {published data only}
Walter 1983 {published data only}
-
- Walter T, Kovalskys J, Stekel A. Effect of mild iron deficiency on infant mental development scores. Journal of Pediatrics 1983;102(4):519‐22. - PubMed
Additional references
AHRQ 2006
-
- Agency for Healthcare Research and Quality. Screening for iron deficiency anemia in childhood and pregnancy. Publication No. AHRQ 06‐0590. - PubMed
Bayley 1993
-
- Bayley N. Bayley Scales of Infant Development. 2nd Edition. San Antonio: The Psychological Corporation, 1993.
Dallman 1975
-
- Dallman PR, Simes M, Manies EC. Brain iron: persistent deficiency following short term iron deprivation in the young rat. British Journal of Haematology 1975;31(2):209‐15. - PubMed
De Maeyer 1985
-
- Maeyer E, Adiels‐Tegman M. The prevalence of anaemia in the world. World Health Statistics Quarterly 1985;38(3):302‐16. - PubMed
Felt 1996
-
- Felt BT, Lozoff B. Brain iron and behaviour of rats are not normalised by treatment of iron deficiency anaemia during early development. Journal of Nutrition 1996;126(3):693‐701. - PubMed
Follmann 1992
-
- Follmann D, Elliott P, Suh I, Cutler J. Variance imputation for overviews of clinical trials with continuous response. Journal of Clinical Epidemiology 1992;45(7):769‐73. - PubMed
Goddard 2011
-
- Goddard AF, James MW, Mclntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut 2011;60(10):1309‐16. - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lansdown 1995
-
- Lansdown R, Wharton BA. Iron and mental and motor behaviour in children. Iron, Nutrition and Physiological Significance: Report of the British Nutrition Foundation Task Force. London: Chapman and Hall, 1995:65‐78.
Larkin 1990
-
- Larkin EC, Rao GA. Importance of fetal and neonatal iron: adequacy for normal development of the central nervous system. In: Dobbing J editor(s). Brain, Behaviour and Iron in the Infant Diet. London: Springer‐Verlag, 1990:43‐62.
Lee 1993
-
- Lee GR. Wintrobe's Clinical Hematology. 9th Edition. Vol. 1, Philadelphia, PA: Lea & Febiger, 1993.
Logan 1997
Lozoff 1986
-
- Lozoff B, Brittenham GM. Behavioural aspects of iron deficiency. Progress in Haematology 1986;XIV:23‐53. - PubMed
Martin 2001
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Roncagliolo 1998
-
- Roncagliolo M, Garrido M, Walter T, Peirano P, Lozoff B. Evidence of altered central nervous system development in infants with iron deficiency anaemia at 6 months: delayed maturation of auditory brainstem responses. The American Journal of Clinical Nutrition 1998;68(3):683‐90. - PubMed
Sachdev 2004
-
- Sachdev HPS, Gera T, Nestel P. Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutrition 2004;8(2):117‐32. - PubMed
Szajewska 2010
-
- Szajewska H, Ruszczynski M, Chmielewska A. Effects of iron supplementation in nonanemic pregnant women, infants, and young children on the mental performance and psychomotor development of children: a systematic review of randomized controlled trials. The American Journal of Clinical Nutrition 2010;91(6):1684‐90. - PubMed
Walter 1994
-
- Walter T. Effect of iron‐deficiency anaemia on cognitive skills in infancy and childhood. Bailliere's Clinical Haematology 1994;7(4):815‐27. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

