Ready-to-use therapeutic food for home-based treatment of severe acute malnutrition in children from six months to five years of age
- PMID: 23744450
- PMCID: PMC6478236
- DOI: 10.1002/14651858.CD009000.pub2
Ready-to-use therapeutic food for home-based treatment of severe acute malnutrition in children from six months to five years of age
Update in
-
Ready-to-use therapeutic food (RUTF) for home-based nutritional rehabilitation of severe acute malnutrition in children from six months to five years of age.Cochrane Database Syst Rev. 2019 May 15;5(5):CD009000. doi: 10.1002/14651858.CD009000.pub3. Cochrane Database Syst Rev. 2019. PMID: 31090070 Free PMC article.
Abstract
Background: Malnourished children have a higher risk of death and illness. Treating severe acute malnourished children in hospitals is not always desirable or practical in rural settings, and home treatment may be better. Home treatment can be food prepared by the carer, such as flour porridge, or commercially manufactured food such as ready-to-use therapeutic food (RUTF). RUTF is made according to a standard, energy-rich composition defined by the World Health Organization (WHO). The benefits of RUTF include a low moisture content, long shelf life without needing refrigeration and that it requires no preparation.
Objectives: To assess the effects of home-based RUTF on recovery, relapse and mortality in children with severe acute malnutrition.
Search methods: We searched the following electronic databases up to April 2013: Cochrane Central Register of Clinical Trials (CENTRAL), MEDLINE, MEDLINE In-process, EMBASE, CINAHL, Science Citation Index, African Index Medicus, LILACS, ZETOC and three trials registers. We also contacted researchers and clinicians in the field and handsearched bibliographies of included studies and relevant reviews.
Selection criteria: We included randomised and quasi-randomised controlled trials where children between six months and five years of age with severe acute malnutrition were treated at home with RUTF compared to a standard diet, or different regimens and formulations of RUTFs compared to each other. We assessed recovery, relapse and mortality as primary outcomes, and anthropometrical changes, time to recovery and adverse outcomes as secondary outcomes.
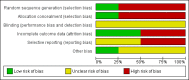
Data collection and analysis: Two review authors independently assessed trial eligibility using prespecified criteria, and three review authors independently extracted data and assessed trial risk of bias.
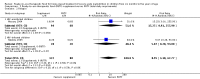
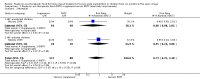
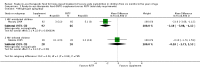
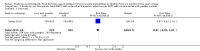
Main results: We included four trials (three having a high risk of bias), all conducted in Malawi with the same contact author. One small trial included children infected with human immunodeficiency virus (HIV). We found the risk of bias to be high for the three quasi-randomised trials while the fourth trial had a low to moderate risk of bias. Because of the sparse data for HIV, we reported below the main results for all children together. RUTF meeting total daily requirements versus standard dietWhen comparing RUTF with standard diet (flour porridge), we found three quasi-randomised cluster trials (n = 599). RUTF may improve recovery slightly (risk ratio (RR) 1.32; 95% confidence interval (CI) 1.16 to 1.50; low quality evidence), but we do not know whether RUTF improves relapse, mortality or weight gain (very low quality evidence). RUTF supplement versus RUTF meeting total daily requirementsWhen comparing RUTF supplement with RUTF that meets total daily nutritional requirements, we found two quasi-randomised cluster trials (n = 210). For recovery, relapse, mortality and weight gain the quality of evidence was very low; therefore, the effects of RUTF are unknown. RUTF containing less milk powder versus standard RUTFWhen comparing a cheaper RUTF containing less milk powder (10%) versus standard RUTF (25% milk powder), we found one trial that randomised 1874 children. For recovery, there was probably little or no difference between the groups (RR 0.97; 95% CI 0.93 to 1.01; moderate quality evidence). RUTF containing less milk powder may lead to slightly more children relapsing (RR 1.33; 95% CI 1.03 to 1.72; low quality evidence) and to less weight gain (mean difference (MD) -0.5 g/kg/day; 95% CI -0.75 to -0.25; low-quality evidence) than standard RUTF. We do not know whether the cheaper RUTF improved mortality (very low quality evidence).
Authors' conclusions: Given the limited evidence base currently available, it is not possible to reach definitive conclusions regarding differences in clinical outcomes in children with severe acute malnutrition who were given home-based ready-to-use therapeutic food (RUTF) compared to the standard diet, or who were treated with RUTF in different daily amounts or formulations. For this reason, either RUTF or flour porridge can be used to treat children at home depending on availability, affordability and practicality. Well-designed, adequately powered pragmatic randomised controlled trials of HIV-uninfected and HIV-infected children with severe acute malnutrition are needed.
Conflict of interest statement
Anel Schoonees ‐ none known.
Martani Lombard ‐ none known.
Alfred Musekiwa ‐ none known.
Etienne Nel ‐ none known.
Jimmy Volmink ‐ none known.
Figures
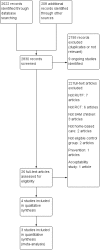


























References
References to studies included in this review
-
- Ciliberto MA, Sandige H, Ndekha MJ, Ashorn P, Briend A, Ciliberto HM, et al. Comparison of home‐based therapy with ready‐to‐use therapeutic food with standard therapy in the treatment of malnourished Malawian children: a controlled, clinical effectiveness trial. The American Journal of Clinical Nutrition 2005;81(4):864‐70. - PubMed
-
- Ndekha MJ, Manary MJ, Ashorn P, Briend A. Home‐based therapy with ready‐to‐use therapeutic food is of benefit to malnourished, HIV‐infected Malawian children. Acta Paediatrica 2005;94(2):222‐5. - PubMed
References to studies excluded from this review
-
- Amthor RE, Cole SM, Manary MJ. The use of home‐based therapy with ready‐to‐use therapeutic food to treat malnutrition in a rural area during a food crisis. Journal of the American Dietetic Association 2009;109(3):464‐7. - PubMed
-
- Briend A, Lacsala R, Prudhon C, Mounier B, Grellety Y, Golden MHN. Ready‐to‐use therapeutic food for treatment of marasmus. Lancet 1999;353(9166):1767‐8. - PubMed
-
- Diop EHI, Dossou NI, Ndour MM, Briend A, Wade S. Comparison of the efficacy of a solid ready‐to‐use food and a liquid, milk‐based diet for the rehabilitation of severely malnourished children: a randomized trial. The American Journal of Clinical Nutrition 2003;78(2):302‐7. - PubMed
-
- Diop EI, Dossou NI, Briend A, Yaya MM, Ndour MM, Wade S. Home‐based rehabilitation for severely malnourished children using locally made ready‐to‐use therapeutic food (RTUF). Proceedings of the Pediatric Gastroenterology, Hepatology and Nutrition 2nd World Congress; 2004 Jul 3‐7; Paris. 2004.
-
- Dube B, Rongsen T, Mazumder S, Taneja S, Rafiqui F, Bhandari N, et al. Comparison of ready‐to‐use therapeutic food with cereal legume‐based khichri among malnourished children. Indian Pediatrics 2009;46(5):383‐8. - PubMed
References to ongoing studies
-
- CTRI/2011/12/002259. Effectiveness of ready to use therapeutic food (RUTF) in community based management of uncomplicated severe acute malnutrition (SAM) in an urban resettlement area in Chandigarh: a randomized controlled trial. apps.who.int/trialsearch/trial.aspx?trialid=CTRI/2011/12/002259 (accessed 20 May 2013).
-
- CTRI/2012/10/003054. To evaluate the impact of three feeding regimens on the recovery of children from uncomplicated severe acute malnutrition (SAM) in India and to use the evidence to inform national policy. apps.who.int/trialsearch/trial.aspx?trialid=CTRI/2012/10/003054 (accessed 20 May 2013).
-
- ISRCTN62376241. Acceptability, effectiveness and cost‐effectiveness of soya maize sorghum‐based ready‐to‐use therapeutic food in treating severe acute malnutrition in children under five in Lusaka, Zambia. www.controlled‐trials.com/ISRCTN62376241 (accessed 10 May 2013).
-
- NCT00131417. Comparison of the efficacy of a ready‐to‐use therapeutic food with a milk‐based diet in the rehabilitation of severely malnourished Ugandan children. www.clinicaltrials.gov/ct2/show/NCT00131417 (accessed 20 May 2013).
-
- NCT00941434. Community based management of malnutrition. A proposal for Pakistan Initiative for Mothers and Newborns. www.clinicaltrials.gov/ct2/show/NCT00941434 (accessed 20 May 2013).
Additional references
-
- ACF International Network. Action against hunger: acute malnutrition ‐ a preventable pandemic. www.actionagainsthunger.org (accessed 20 May 2013).
-
- Ashworth A. Efficacy and effectiveness of community‐based treatment of severe malnutrition. Food and Nutrition Bulletin 2006;27(3 Suppl):S24‐8. - PubMed
-
- Bhutta ZA, Ahmed T, Black RE, Cousens S, Dewey K, Giugliani E, et al. What works? Interventions for maternal and child undernutrition and survival. Lancet 2008;371(9610):417‐40. - PubMed
-
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 2008;371(9608):243‐60. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

