Building reactive copper centers in human carbonic anhydrase II
- PMID: 23744511
- PMCID: PMC4059033
- DOI: 10.1007/s00775-013-1009-1
Building reactive copper centers in human carbonic anhydrase II
Abstract
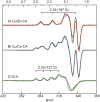
Reengineering metalloproteins to generate new biologically relevant metal centers is an effective a way to test our understanding of the structural and mechanistic features that steer chemical transformations in biological systems. Here, we report thermodynamic data characterizing the formation of two type-2 copper sites in carbonic anhydrase and experimental evidence showing one of these new, copper centers has characteristics similar to a variety of well-characterized copper centers in synthetic models and enzymatic systems. Human carbonic anhydrase II is known to bind two Cu(2+) ions; these binding events were explored using modern isothermal titration calorimetry techniques that have become a proven method to accurately measure metal-binding thermodynamic parameters. The two Cu(2+)-binding events have different affinities (K a approximately 5 × 10(12) and 1 × 10(10)), and both are enthalpically driven processes. Reconstituting these Cu(2+) sites under a range of conditions has allowed us to assign the Cu(2+)-binding event to the three-histidine, native, metal-binding site. Our initial efforts to characterize these Cu(2+) sites have yielded data that show distinctive (and noncoupled) EPR signals associated with each copper-binding site and that this reconstituted enzyme can activate hydrogen peroxide to catalyze the oxidation of 2-aminophenol.
Figures

References
-
- Lu Y, Berry SM, Pfister TD. Chem Rev. 2001;101:2047–3080. - PubMed
-
- Håkansson K, Wehnert A, Liljas A. Acta Crystallogr D Biol Crystallogr. 1994;50:93–100. - PubMed
-
- Hunt JB, Rhee MJ, Storm CB. Anal Biochem. 1977;79:614–617. - PubMed
-
- Cox JD, Hunt JA, Compher KM, Fierke CA, Christianson DW. Biochemistry. 2000;39:13687–13694. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

