Fluoroquinolones for treating tuberculosis (presumed drug-sensitive)
- PMID: 23744519
- PMCID: PMC6532730
- DOI: 10.1002/14651858.CD004795.pub4
Fluoroquinolones for treating tuberculosis (presumed drug-sensitive)
Abstract
Background: Currently the World Health Organization only recommend fluoroquinolones for people with presumed drug-sensitive tuberculosis (TB) who cannot take standard first-line drugs. However, use of fluoroquinolones could shorten the length of treatment and improve other outcomes in these people. This review summarises the effects of fluoroquinolones in first-line regimens in people with presumed drug-sensitive TB.
Objectives: To assess fluoroquinolones as substitute or additional components in antituberculous drug regimens for drug-sensitive TB.
Search methods: We searched the Cochrane Infectious Diseases Group Specialized Register; CENTRAL (The Cochrane Library 2013, Issue 1); MEDLINE; EMBASE; LILACS; Science Citation Index; Databases of Russian Publications; and metaRegister of Controlled Trials up to 6 March 2013.
Selection criteria: Randomized controlled trials (RCTs) of antituberculous regimens based on rifampicin and pyrazinamide and containing fluoroquinolones in people with presumed drug-sensitive pulmonary TB.
Data collection and analysis: Two authors independently applied inclusion criteria, assessed the risk of bias in the trials, and extracted data. We used the risk ratio (RR) for dichotomous data and the fixed-effect model when it was appropriate to combine data and no heterogeneity was present. We assessed the quality of evidence using the GRADE approach.
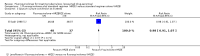
Main results: We identified five RCTs (1330 participants) that met the inclusion criteria. None of the included trials examined regimens of less than six months duration. Fluoroquinolones added to standard regimensA single trial (174 participants) added levofloxacin to the standard first-line regimen. Relapse and treatment failure were not reported. For death, sputum conversion, and adverse events we are uncertain if there is an effect (one trial, 174 participants, very low quality evidence for all three outcomes). Fluoroquinolones substituted for ethambutol in standard regimens Three trials (723 participants) substituted ethambutol with moxifloxacin, gatifloxacin, and ofloxacin into the standard first-line regimen. For relapse, we are uncertain if there is an effect (one trial, 170 participants, very low quality evidence). No trials reported on treatment failure. For death, sputum culture conversion at eight weeks, or serious adverse events we do not know if there was an effect (three trials, 723 participants, very low quality evidence for all three outcomes). Fluoroquinolones substituted for isoniazid in standard regimens A single trial (433 participants) substituted moxifloxacin for isoniazid. Treatment failure and relapse were not reported. For death, sputum culture conversion, or serious adverse events the substitution may have little or no difference (one trial, 433 participants, low quality evidence for all three outcomes). Fluoroquinolines in four month regimensSix trials are currently in progress testing shorter regimens with fluoroquinolones.
Authors' conclusions: Ofloxacin, levofloxacin, moxifloxacin, and gatifloxacin have been tested in RCTs of standard first-line regimens based on rifampicin and pyrazinamide for treating drug-sensitive TB. There is insufficient evidence to be clear whether addition or substitution of fluoroquinolones for ethambutol or isoniazid in the first-line regimen reduces death or relapse, or increases culture conversion at eight weeks. Much larger trials with fluoroquinolones in short course regimens of four months are currently in progress.
Conflict of interest statement
GDAV is a co‐author on the trial report of Rustomjee 2008a.
Figures















Update of
-
Fluoroquinolones for treating tuberculosis.Cochrane Database Syst Rev. 2008 Jan 23;(1):CD004795. doi: 10.1002/14651858.CD004795.pub3. Cochrane Database Syst Rev. 2008. Update in: Cochrane Database Syst Rev. 2013 Jun 06;(6):CD004795. doi: 10.1002/14651858.CD004795.pub4. PMID: 18254061 Updated.
References
References to studies included in this review
Burman 2006 {published data only}
-
- Burman WJ, Goldberg S, Johnson JL, Muzanye G, Engle M, Mosher AW, et al. Moxifloxacin versus ethambutol in the first two months of treatment for pulmonary tuberculosis. American Journal of Respiratory and Critical Care Medicine 2006;174(3):331‐8. - PubMed
Conde 2009 {published data only}
Dorman 2009 {published data only}
-
- Dorman SE, Johnson JL, Goldberg S, Muzanye G, Padayatchi N, Bozeman L, et al. Substitution of moxifloxacin for isoniazid during intensive phase treatment of pulmonary tuberculosis. American Journal of Respiratory and Critical Care Medicine 2009;180(3):273‐80. - PubMed
El‐Sadr 1998 {published data only}
-
- El‐Sadr WM, Perlman DC, Matts JP, Nelson ET, Cohn DL, Salomon N, et al. Evaluation of an intensive intermittent‐induction regimen and duration of short‐course treatment for human immunodeficiency virus‐related pulmonary tuberculosis. Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) and the AIDS Clinical Trials Group (ACTG). Clinical Infectious Diseases 1998;26(5):1148‐58. - PubMed
Rustomjee 2008a {published data only}
-
- Rustomjee R, Lienhardt C, Kanyok T, Davies GR, Levin J, Mthiyane T, et al. A Phase II study of the sterilising activities of ofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease 2008;12(2):128‐38. - PubMed
References to studies excluded from this review
Abdullah 1997 {published data only}
-
- Abdullah A, Abu‐Hussein AH, Al‐Akshar M, Abdel R, Ads CH, Taieb S. Ofloxacin in the treatment of primary drug resistant pulmonary tuberculosis (PDR‐TB). European Respiratory Journal 1997;10(Suppl):25214.
Abdullah 1998 {published data only}
-
- Abdullah A, Abu‐Hussein AH, Al‐Akshar M, Ads CH, El‐Taieb S. Ofloxacin dosage in the treatment of multidrug resistant pulmonary tuberculosis (MDR‐TB). European Respiratory Journal 1998;12(Suppl):28367.
Agarwal 2007 {published data only}
-
- Agarwal SK. Treatment of resistant tuberculosis in Indian patients of Eastern Uttar Pradesh having isolates resistant to isoniazid, rifampicin and quinolones. Respirology 2007;12(4):A114.
Andries 2005 {published data only}
-
- Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs LM, Winkler H, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005;307(5707):223‐7. - PubMed
Anonymous 1997 {published data only}
-
- Anonymous. A controlled study of rifabutin and an uncontrolled study of ofloxacin in the retreatment of patients with pulmonary tuberculosis resistant to isoniazid, streptomycin and rifampicin. Hong Kong Chest Service/British Medical Research Council. Tubercle and Lung Disease 1997;73(1):59‐67. - PubMed
Barmina 2009 {published data only}
-
- Barmina NA, Burukhina LV, Shurygin AA, Archakova LI. Roncoleukin in enhancing the efficiency of complex therapy for infiltrative pulmonary tuberculosis in adolescents. Problemy Tuberkuleza i Boleznei Legkikh 2009;5:27‐31. - PubMed
Bartacek 2009 {published data only}
-
- Bartacek A, Schutt D, Panosch B, Borek M, Rimstar 4‐FDC Study Group. Comparison of a four‐drug fixed‐dose combination regimen with a single tablet regimen in smear‐positive pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease 2009;13(6):760‐6. - PubMed
Carroll 2012 {published and unpublished data}
Chambers 1998 {published data only}
-
- Chambers HF, Kocagoz T, Sipit T, Turner J, Hopewell PC. Activity of amoxicillin/clavulanate in patients with tuberculosis. Clinical Infectious Diseases 1998;26(4):874‐7. - PubMed
Chang 2008 {published data only}
-
- Chang KC, Leung CC, Yew WW, Lau TY, Tam CM. Hepatotoxicity of pyrazinamide: Cohort and case‐control analyses. American Journal of Respiratory and Critical Care Medicine 2008;177(12):1391‐6. - PubMed
Chang 2009 {published data only}
-
- Chang KC, Leung CC, Yew WW, Lau TY, Leung WM, Tam CM, et al . Newer fluoroquinolones for treating respiratory infection: do they mask tuberculosis?. European Respiratory Journal 2009;35(3):606‐13. - PubMed
Chen 2003 {published data only}
-
- Chen QL, Chen L, Yin JJ. A study on the clinical efficacy of a combination regimen with levofloxacin and capreomycin in the treatment of multi‐drug resistant pulmonary tuberculosis. Zhonghua Jie He He Hu Xi Za Zhi [Chinese Journal of Tuberculosis and Respiratory Diseases] 2003;26(8):454‐7. - PubMed
Chigutsa 2012 {published and unpublished data}
Chukanov 2006 {published data only}
-
- Chukanov VI, Komissarova OG, Maishin VI, Abdullaev RI, Kononets AS. Efficiency of a new standard chemotherapy regimen in the treatment of patients with recurrent pulmonary tuberculosis. Problemy Tuberculeza i Boleznej Legkikh 2006, issue 8:9‐13. - PubMed
Diacon 2009 {published data only}
-
- Diacon AH, Pym A, Grobusch M, Patientia R, Rustomjee R, Page‐Shipp L, et al. The diarylquinoline TMC207 for multidrug‐resistant tuberculosis. New England Journal of Medicine 2009;360(23):2397‐405. - PubMed
Diacon 2012a {published and unpublished data}
-
- Diacon AH, Donald PR, Pym A, Grobusch M, Patientia RF, Mahanyele R, et al. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug‐resistant tuberculosis: long‐term outcome, tolerability and effect on emergence of drug resistance. Antimicrobial Agents and Chemotherapy 2012;56(6):3271‐6. - PMC - PubMed
Diacon 2012b {published data only}
-
- Diacon AH, Dawson R, Groote‐Bidlingmaier F, Symons G, Venter A, Donald PR, et al. 14‐day bactericidal activity of PA‐824, bedaquiline, pyrazinamide, and moxifloxacin combinations: a randomised trial. Lancet 2012;380(9846):986‐93. - PubMed
Estebanez 1992 {published data only}
-
- Estebanez Zarranz MJ, Martinez‐Sagarra JM, Alberte A, Amon Sesmero J, Rodriguez Toves A. Treatment of urogenital tuberculosis with ofloxacin. Preliminary study. Actas Urologicas Espanolas 1992;16(1):64‐8. - PubMed
Fouad 2011 {published and unpublished data}
-
- Fouad M, Gallagher JC. Moxifloxacin as an alternative or additive therapy for treatment of pulmonary tuberculosis. Annals of Pharmacotherapy 2011;45(11):1439‐44. - PubMed
Gosling 2003 {published data only}
-
- Gosling RD, Uiso LO, Sam NE, Bongard E, Kanduma EG, Nyindo M, et al. The bactericidal activity of moxifloxacin in patients with pulmonary tuberculosis. American Journal of Respiratory and Critical Care Medicine 2003;168(11):1342‐5. - PubMed
Grishin 1998 {published data only}
-
- Grishin VK, Polunina TE. Lomefloxacin in phthisiatric practice. Antibiotiki i Khimioterapiia 1998;43(10):17‐8. - PubMed
Heemskerk 2011 {published and unpublished data}
Ho 2007 {published data only}
-
- Ho CC, Chen YC, Liao WY, Tsai TH, YU CJ, Yang PC, et al. Safety of fluoroquinolone in the treatment of tuberculosis patients with hepatotoxicity induced by first‐line antituberculosis regimens [Abstract]. Respirology 2007;12((Suppl 4)):A240.
Huang 2000 {published data only}
-
- Huang CS, Wu CC. Observation of the clinical efficacy of sparfloxacin in the treatment of multiple drug resistance pneumonial tuberculosis. Chinese Journal of Antibiotics 2000;25(4):302‐3.
Jenkins 2008 {published data only}
-
- Jenkins PA, Campbell IA, Banks J, Gelder CM, Prescott RJ, Smith AP. Clarithromycin vs ciprofloxacin as adjuncts to rifampicin and ethambutol in treating opportunist mycobacterial lung diseases and an assessment of Mycobacterium vaccae immunotherapy. Thorax 2008;63(7):627‐34. - PubMed
Ji 2001 {published data only}
-
- Ji YM, Dong LH, Wang Q, Yu WQ. Short‐term observating of curative effects in treatment of multiple‐drug resistance pulmonary tuberculosis with sparfloxacin and ofloxacin. Journal of Postgraduates of Medicines 2001;24(7):32‐3.
Johnson 2006 {published data only}
-
- Johnson JL, Hadad DJ, Boom WH, Daley CL, Peloquin CA, Eisenach KD, et al. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. International Journal of Tuberculosis and Lung Disease 2006;10(6):605‐12. - PubMed
Kang 2009 {published data only}
-
- Kang WL, Xie YG, Tan WG, Chu NH, Li L, You YH, et al. Study on the efficacy and safety of short‐term treatment including fluoroquinolones anti‐tuberculosis drugs for rifampicin resistant pulmonary tuberculosis. Zhonghua Liu Xing Bing Xue Za Zhi 2009;30(2):179‐83. - PubMed
Kawahara 1992 {published data only}
-
- Kawahara S, Eirei J. Evaluation of new antitubercular agents‐ new quinolone agents. Kekkaku 1992;67(10):679‐82. - PubMed
Kennedy 1993 {published data only}
-
- Kennedy N, Fox R, Uiso L, Ngowi FI, Gillespie SH. Safety profile of ciprofloxacin during long‐term therapy for pulmonary tuberculosis. Journal of Antimicrobial Chemotherapy 1993;32(6):897‐902. - PubMed
Kennedy 1996 {published data only}
-
- Kennedy N, Berger L, Curram J, Fox R, Gutmann J, Kisyombe GM, et al. Randomized controlled trial of a drug regimen that includes ciprofloxacin for the treatment of pulmonary tuberculosis. Clinical Infectious Diseases 1996;22(5):827‐33. - PubMed
-
- Kennedy N, Fox R, Kisyombe GM, Saruni AO, Uiso LO, Ramsay AR, et al. Early bactericidal and sterilizing activities of ciprofloxacin in pulmonary tuberculosis. American Review of Respiratory Disease 1993;148(6 Pt 1):1547‐51. - PubMed
Kohno 1992 {published data only}
-
- Kohno S, Koga H, Kaku M, Maesaki S, Hara K. Prospective comparative study of ofloxacin or ethambutol for the treatment of pulmonary tuberculosis. Chest 1992;102(6):1815‐8. - PubMed
Kumar 2004 {published data only}
-
- Kumar AK, Gurumurthy P. Disposition of uric acid upon administration of ofloxacin alone and in combination with other anti‐tuberculosis drugs. Indian Journal of Experimental Biology 2004;42(3):323‐5. - PubMed
Lee 2011 {published and unpublished data}
Li 2008 {published data only}
-
- Li L, Zheng SH, Chu NH, Xie YG, Yang YZ, Li Q, et al. Effects of two treatment regimens for drug‐resistant tuberculosis in tuberculosis control project areas: a comparative study. Zhonghua Yi Xue Za Zhi 2008;88(48):3387‐91. - PubMed
Lu 2000 {published data only}
-
- Lu Y, Zhu L, Duan L. Antituberculosis effect of levofloxacin. Zhonghua Jie He He Hu Xi Za Zhi 2000;23(1):50‐4. - PubMed
Marra 2005 {published data only}
-
- Marra F, Marra CA, Moadebi S, Shi P, Elwood RK, Stark G, et al. Levofloxacin treatment of active tuberculosis and the risk of adverse events. Chest 2005;128(3):1406‐13. - PubMed
Merle 2012 {published data only (unpublished sought but not used)}
-
- Merle CS, Sismanidis C, Sow OB, Gninafon M, Horton J, Lapujade O, et al. A pivotal registration phase III, multicenter, randomized tuberculosis controlled trial: design issues and lessons learnt from the Gatifloxacin for TB (OFLOTUB) project. Trials 2012; Vol. 13, issue 61:1‐10. [DOI: 10.1186/1745-6215-13-61] - DOI - PMC - PubMed
Moadebi 2007 {published data only}
-
- Moadebi S, Harder CK, Fitzgerald MJ, Elwood KR, Marra F. Fluoroquinolones for the treatment of pulmonary tuberculosis. Drugs 2007;67(14):2077‐99. - PubMed
Mohanty 1993 {published data only}
-
- Mohanty KC, Dhamgaye TM. Controlled trial of ciprofloxacin in short‐term chemotherapy for pulmonary tuberculosis. Chest 1993;104(4):1194‐8. - PubMed
Moulding 2008 {published data only}
-
- Moulding T. A randomised controlled trial of high‐dose isoniazid adjuvant therapy for multidrug‐resistant tuberculosis. International Journal of Tuberculosis and Lung Disease 2008;12(9):1102. - PubMed
Nakamura 2007 {published data only}
-
- Nakamura M, Hasegawa N, Miyao N, Nakajima T, Terashima T, Sakamaki F, et al. Comparison of five day short‐course therapies for secondary infection in patients with chronic respiratory disease using gatifloxacin and levofloxacin. Japanese Journal of Chemotherapy 2007;55(6):451‐62.
Nosova 2008 {published data only}
-
- Nosova EIu, Galkina KIu, Antonova OV, Garmash IuIu, Skotnikova OI, Moroz AM. Use of molecular‐biological microchip TB‐BIOCHIP‐2 for detecting of Mycobacterium tuberculosis with multidrug resistance to fluoroquinolones in patients with new detected and chronic tuberculosis. Vestnik Rossiiskoi Akademii Meditsinskikh Nauk 2008;3:16‐9. - PubMed
O'Brien 1994 {published data only}
-
- O'Brien RJ, Nunn PP. Ciprofloxacin is not a component of first‐line TB. Chest 1994;106(4):1312. - PubMed
Peloquin 2008 {published data only}
Pletz 2004 {published data only}
Ruslami 2013 {published data only}
-
- Ruslami R, Ganiem AR, Dian S, Apriani L, Achmad TH, Ven AJ, et al. Intensified regimen containing rifampicin and moxifloxacin for tuberculous meningitis: an open‐label, randomised controlled phase 2 trial. Lancet Infectious Diseases 2013;13(1):27‐35. - PubMed
Rustomjee 2008b {published data only}
Saigal 2001 {published data only}
-
- Saigal S, Agarwal SR, Nandeesh HP, Sarin SK. Safety of an ofloxacin‐based antitubercular regimen for the treatment of tuberculosis in patients with underlying chronic liver disease: a preliminary report. Journal of Gastroenterology and Hepatology 2001;16(9):1028‐32. - PubMed
Sirgel 1997 {published data only}
-
- Sirgel FA, Botha FJ, Parkin DP, Wal BW, Schall R, Donald PR, et al. The early bactericidal activity of ciprofloxacin in patients with pulmonary tuberculosis. American Journal of Respiratory and Critical Care Medicine 1997;156(3 Pt 1):901‐5. - PubMed
Sirgel 2000 {published data only}
-
- Sirgel FA, Donald PR, Odhiambo J, Githui W, Umapathy KC, Paramasivan CN, et al. A multicentre study of the early bactericidal activity of anti‐tuberculosis drugs. Journal of Antimicrobial Chemotherapy 2000;45(6):859‐70. - PubMed
Sokolova 1998 {published data only}
-
- Sokolova GB, Kunichan AD, Koriakin VA, Lazareva IaV. Lomefloxacin in complex treatment of acute progressive form of pulmonary tuberculosis. Antibiotiki i Khimioterapiia 1998;43(10):10‐2. - PubMed
Sun 2000 {published data only}
-
- Sun W, Wenyi C, Cunzhi L, Yanhong Y, Yuzhen X, Zhaosheng S. A randomized controlled study of sparfloxacin and ofloxacin in the treatment of multiple drug resistant pulmonary tuberculosis. Chinese Journal of Antibiotics 2000;25(1):52‐4.
Suo 1996 {published data only}
-
- Suo J, Yu MC, Lee CN, Chiang CY, Lin TP. Treatment of multidrug‐resistant tuberculosis in Taiwan. Chemotherapy 1996;42(Suppl 3):20‐3. - PubMed
Thwaites 2011 {published and unpublished data}
TRC 2002 {published data only}
-
- Tuberculosis Research Centre (Indian Council of Medical Research), Chennei. Shortening short course chemotherapy: a randomized clinical trial for treatment of smear positive pulmonary tuberculosis with regimens using ofloxacin in the intensive phase. Indian Journal of Tuberculosis 2002;49(1):27‐38.
Valerio 2003 {published data only}
-
- Valerio G, Bracciale P, Manisco V, Quitadamo M, Legari G, Bellanova S. Long‐term tolerance and effectiveness of moxifloxacin therapy for tuberculosis: preliminary results. Journal of Chemotherapy 2003;15(1):66‐70. - PubMed
Venter 2006 {published data only}
-
- Venter WDF, Panz VR, Feldman Ch, Joffe BI. Adrenocortical function in hospitalised patients with active pulmonary tuberculosis receiving a rifampicin‐based regimen ‐ a pilot study. South African Medical Journal 2006;96(1):62‐6. - PubMed
Wang 2006 {published data only}
Wolbers 2011 {published and unpublished data}
-
- Wolbers M, Heemskerk D, Chau TT, Yen NT, Caws M, Farrar J, et al. Sample size requirements for separating out the effects of combination treatments: randomised controlled trials of combination therapy vs. standard treatment compared to factorial designs for patients with tuberculous meningitis. Trials 2011;12(26):1‐7. - PMC - PubMed
Yoon 2005 {published data only}
-
- Yoon YS, Lee HJ, Yoon HI, Yoo CG, Kim YW, Han SK, et al. Impact of fluoroquinolones on the diagnosis of pulmonary tuberculosis initially treated as bacterial pneumonia. International Journal of Tuberculosis and Lung Disease 2005;9(11):1215‐9. - PubMed
Yoon 2012 {published and unpublished data}
-
- Yoon HE, Jeon YJ, Chung HW, Shin SJ, Hwang HS, Lee SJ, et al. Safety and efficacy of a quinolone‐based regimen for treatment of tuberculosis in renal transplant recipients. Transplantation Proceedings 2012;44(3):730‐3. - PubMed
Zhang 1997 {published data only}
-
- Zhang Y, Qian H, Chen M. Bronchofiberscope and catheter intervention in treatment of multi‐drug resistant pulmonary tuberculosis. Zhonghua Jie He He Hu Xi Za Zhi 1997;20(6):354‐7. - PubMed
Zhang 2006 {published data only}
-
- Zhang X, Li M, Hu CM, Yu J, Lin FS, Mao KJ, et al. Clinic assessment of rifabutin in the treatment of multidrug‐resistant pulmonary tuberculosis. Chinese Journal of Antibiotics 2006;31(4):223‐4, 242.
Zhao 2003 {published data only}
-
- Zhao RZ, Wang QM, Zhang PC, Zhang HM. Therapeutic effects of levofloxacin‐containing regimen in patients with retreated pulmonary tuberculosis. Chinese Journal of Antibiotics 2003;28(8):497‐9.
Zheng 2004 {published data only}
-
- Zheng XM, Li SM, Xing BC. Short‐term effect of treatment protocol utilizing levofloxacin, pasiniazide and M. vaccae on multi‐drug resistant pulmonary tuberculosis. Di Yi Jun Yi Da Xue Xue Bao [Academic Journal of the First Medical College of PLA] 2004;24(5):574‐5. - PubMed
Zhu 2006 {published data only}
-
- Zhu LZ, Fu Y, Chu NH, Ye ZZ, Xiao HP, Wang W, et al. A controlled clinical trial of long course chemotherapy regimens containing rifabutin in the treatment of multi‐drug resistant pulmonary tuberculosis. Zhonghua Jie He He Hu Xi Za Zhi 2006;29(8):520‐3. - PubMed
Zhu 2012 {published and unpublished data}
-
- Zhu H, Lei X, Zhang F, Zhang ZJ, Lu J, Li H, et al. Effectiveness and safety of levofloxacin for multidrug resistant pulmonary tuberculosis: A systematic review. Chinese Journal of Evidence‐Based Medicine 2012;12(2):201‐8.
Zvada 2012 {published and unpublished data}
References to ongoing studies
CTRI/2012/10/003060 {published data only}
-
- ICMR. Thrice weekly 4‐months moxifloxacin or gatifloxacin regimens for pulmonary TB [A study of the efficacy and tolerability of moxifloxacin and gatifloxacin containing regiments in the treatment of patients with sputum‐positive pulmonary tuberculosis]. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=5124 (accessed in March 2013). - PMC - PubMed
ISRCTN44153044 RIFAQUIN {published data only}
-
- ISRCTN44153044. An international multicentre controlled clinical trial to evaluate high dose RIFApentine and a QUINolone in the treatment of pulmonary tuberculosis. www.controlled‐trials.com/ISRCTN44153044/ISRCTN44153044 (accessed 27 July 2007).
NCT00216385 {published data only}
-
- NCT00216385. A controlled trial of a 4‐month quinolone‐containing regimen for the treatment of pulmonary tuberculosis. clinicaltrials‐nccs.nlm.nih.gov/ct2/show/NCT00216385 (accessed 23 July 2007).
NCT00396084 {published data only}
-
- NCT00396084. Randomized, open label, multiple dose Phase I study of the early bactericidal activity of linezolid, gatifloxacin, levofloxacin, and moxifloxacin in HIV‐non‐infected adults with Initial episodes of sputum smear‐positive pulmonary tuberculosis (DMID 01‐553). clinicaltrials.gov/ct/show/NCT00396084?order=11 (accessed 27 July 2007).
NCT00728507 {published data only}
-
- NCT00728507. A phase II randomized, open‐label trial of a rifapentine plus moxifloxacin‐based regimen for intensive phase treatment of smear‐positive pulmonary tuberculosis. http://clinicaltrials.gov/ct2/show/NCT00728507 (accessed on 9 November 2009).
NCT00864383 REMoxTB {published data only}
-
- NCT00864383. Controlled comparison of two moxifloxacin containing treatment shortening regimens in pulmonary tuberculosis. http://clinicaltrials.gov/ct2/show/NCT00864383 (accessed on 9 November 2009).
NCT01498419 {published data only}
-
- NCT01498419. Evaluation of 8 weeks of treatment with the combination of moxifloxacin, PA‐824 and pyrazinamide in patients With drug sensitive and multi drug‐resistant pulmonary tuberculosis (TB). http://clinicaltrials.gov/ct2/show/NCT01498419 (accessed on 25 May 2012).
NCT01589497 {published data only}
-
- AIDS Clinical Trials Group. Early bactericidal activity (EBA) study of tuberculosis regimens with and without INH and moxifloxacin [Essentiality of isoniazid after the first two doses: a randomized phase IIa clinical trial comparing the early bactericidal activity (EBA) of a standard anti‐tuberculosis regimen (RHZE) with a regimen omitting isoniazid (RZE) or a regimen substituting moxifloxacin for isoniazid (RMZE) during days 3 to 14 of tuberculosis therapy]. http://clinicaltrials.gov/show/NCT01589497 2013.
NCT01785186 {published data only}
-
- Michael Hoelscher. Evaluation of SQ109, high‐dose rifampicin, and moxifloxacin in adults with smear‐positive pulmonary TB in a MAMS Design (PanACEA‐MAMS‐TB‐01) [A multiple arm, multiple stage, phase 2, OL, randomized, controlled trial to evaluate 4 treatment regimens of SQ109, increased doses of rifampicin, and Moxifloxacin in Adults with newly diagnosed, smear‐positive pulmonary tuberculosis]. http://clinicaltrials.gov/ct2/show/NCT01785186 accessed March 2013.
Additional references
Alangaden 1997
-
- Alangaden GJ, Lerner SA. The clinical use of fluoroquinolones for the treatment of mycobacterial diseases. Clinical Infectious Diseases 1997;25(5):1213‐21. - PubMed
Anonymous 1983
-
- Anonymous. Controlled clinical trial of 4 short‐course regimens of chemotherapy (three 6‐month and one 8‐month) for pulmonary tuberculosis. Tubercle 1983;64(3):153‐66. - PubMed
Blumberg 2003
-
- Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. American Journal of Respiratory Critical Care Medicine 2003;167(4):603‐62. - PubMed
Burman 1999
-
- Burman WJ, Gallicano K, Peloquin C. Therapeutic implications of drug interactions in the treatment of human immunodeficiency virus‐related tuberculosis. Clinical Infectious Diseases 1999;28(3):419‐30. - PubMed
Corbett 2003
-
- Corbett EL, Watt CJ, Walker N, Maher D, Williams BG, Raviglione MC, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Archives of Internal Medicine 2003;163(9):1009‐21. - PubMed
Daley 1992
-
- Daley CL, Small PM, Schecter GF, Schoolnik GK, McAdam RA, Jacobs WR Jr, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction‐fragment‐length polymorphisms. New England Journal of Medicine 1992;326(4):231‐5. - PubMed
El‐Sadr 2001
-
- El‐Sadr WM, Perlman DC, Denning E, Marts JP, Cohn DL. A review of efficacy studies of 6‐month short‐course therapy for tuberculosis among patients infected with human immunodeficiency virus: differences in study outcomes. Clinical Infectious Diseases 2001;32(4):623‐32. - PubMed
Falzon 2011
-
- Falzon D, Jaramillo E, Schünemann HJ, Arentz M, Bauer M, Bayona J, et al. WHO guidelines for the programmatic management of drug‐resistant tuberculosis: 2011 update. European Respiratory Journal 2011;38(3):516‐28. - PubMed
Gillespie 1998
-
- Gillespie SH, Kennedy N. Fluoroquinolones: a new treatment for tuberculosis?. International Journal of Tuberculosis and Lung Disease 1998;2(4):265‐71. - PubMed
Ginsburg 2003
-
- Ginsburg AS, Grosset JH, Bishai WR. Fluoroquinolones, tuberculosis, and resistance. Lancet Infectious Diseases 2003;3(7):432‐42. - PubMed
Grzybowski 1975
-
- Grzybowski S, Barnett GD, Styblo K. Contacts of cases of active pulmonary tuberculosis. Bulletin of the International Union Against Tuberculosis 1975; Vol. 50, issue 1:90‐106. - PubMed
Guyatt 2008
Higgins 2011
-
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane.org/resources/handbook/hbook.htm.
Jacobs 1999
-
- Jacobs MR. Activity of quinolones against mycobacteria. Drugs 1999;58(Suppl 2):19‐22. - PubMed
Johnston 2009
Kochi 1991
-
- Kochi A. The global tuberculosis situation and the new control strategy of the World Health Organization. Tubercle 1991;72(1):1‐6. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Search for studies. In: Higgins JPT, Green S (editors) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane.org/resources/handbook/hbook.htm.
Martindale 1996
-
- Reynolds JEF, Parfitt K, Parsons AV, Sweetmann SC, editors. Martindale: the extra pharmacopoeia. 1st Edition. London: Royal Pharmaceutical Society, 1996:1881.
Review Manager 5 [Computer program]
-
- The Nordic Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Centre, The Cochrane Collaboration, 2008.
STS/BMRC 1981
-
- Anonymous. Clinical trial of six‐month and four‐month regimens of chemotherapy in the treatment of pulmonary tuberculosis: the results up to 30 months. Tubercule 1981;62(2):95‐102. - PubMed
Telzak 1999
-
- Telzak EE, Chirgwin KD, Nelson ET, Matts JP, Sepkowitz KA, Benson CA, et al. Predictors for multidrug‐resistant tuberculosis among HIV‐infected patients and response to specific drug regimens. Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) and the AIDS Clinical Trials Group (ACTG), National Institutes for Health. International Journal of Tuberculosis and Lung Disease 1999;3(4):337‐43. - PubMed
WHO 2003
-
- WHO Global Tuberculosis Programme. Treatment of tuberculosis: guidelines for national programmes. [WHO/CDS/TB/2003.313]. 3rd Edition. Geneva: World Health Organization, 2003:1‐13.
WHO 2006
-
- World Health Organization. Stop TB Dept. Guidelines for the programmatic management of drug‐resistant tuberculosis. [WHO/HTM/TB/2006.361]. Geneva: World Health Organization, 2006:38‐53.
WHO 2007a
-
- World Health Organization. WHO model list of essential medicines: 15th edition. WHO Drug Information 2007;21(2):95‐111.
WHO 2007b
-
- World Health Organization. Stop TB Dept. Global tuberculosis control : surveillance, planning, financing : WHO report 2007 [WHO/HTM/TB/2007.376]. http://www.who.int/tb/publications/global_report/2007/download_centre/en.... Geneva: World Health Organization, (accessed August 2007).
WHO 2010a
-
- World Health Organization. Fact File: 10 Facts about tuberculosis. www.who.int/features/factfiles/tb_facts/en/index.html (accessed 8 March 2010).
WHO 2010b
-
- World Health Organisation. Treatment of tuberculosis: guidelines: WHO/HTM/TB/2009.420. 4th Edition. Geneva, Switzerland: World Health Organization, 2009.
WHO 2011a
-
- World Health Organisation. Global tuberculosis control: WHO report 2011. http://www.who.int/tb/publications/global_report/2011/gtbr11_main.pdf 2011:1‐73.
WHO 2011b
-
- World Health Organization. WHO Model list of essential medicines: 17th edition. http://whqlibdoc.who.int/hq/2011/a95053_eng.pdf 2011.
WHO 2012
-
- World Health Organization. Fact sheet No 104: Tuberculosis. http://www.who.int/mediacentre/factsheets/fs104/en/ accessed 25 July 2012.
Woldehanna 2004
Yew 2001
-
- Yew WW. Clinically significant interactions with drugs used in the treatment of tuberculosis. Drug Safety 2001;25(2):111‐33. - PubMed
References to other published versions of this review
Ziganshina 2005
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

