CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia
- PMID: 23744585
- PMCID: PMC3744995
- DOI: 10.1182/blood-2013-02-487074
CMV reactivation after allogeneic HCT and relapse risk: evidence for early protection in acute myeloid leukemia
Abstract
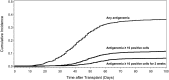
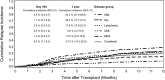
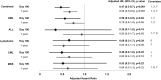
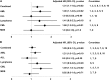
The association between cytomegalovirus (CMV) reactivation and relapse was evaluated in a large cohort of patients with acute myeloid leukemia (AML) (n = 761), acute lymphoblastic leukemia (ALL) (n = 322), chronic myeloid leukemia (CML) (n = 646), lymphoma (n = 254), and myelodysplastic syndrome (MDS) (n = 371) who underwent allogeneic hematopoietic cell transplantation (HCT) between 1995 and 2005. In multivariable models, CMV pp65 antigenemia was associated with a decreased risk of relapse by day 100 among patients with AML (hazard ratio [HR] = 0.56; 95% confidence interval [CI], 0.3-0.9) but not in patients with ALL, lymphoma, CML, or MDS. The effect appeared to be independent of CMV viral load, acute graft-versus-host disease, or ganciclovir-associated neutropenia. At 1 year after HCT, early CMV reactivation was associated with reduced risk of relapse in all patients, but this did not reach significance for any disease subgroup. Furthermore, CMV reactivation was associated with increased nonrelapse mortality (HR = 1.31; 95% CI, 1.1-1.6) and no difference in overall mortality (HR = 1.05; 95% CI, 0.9-1.3). This report demonstrates a modest reduction in early relapse risk after HCT associated with CMV reactivation in a large cohort of patients without a benefit in overall survival.
Figures


Comment in
-
CMV: a warrior against leukemia?Blood. 2013 Aug 15;122(7):1101-2. doi: 10.1182/blood-2013-06-508515. Blood. 2013. PMID: 23950174
References
-
- Lönnqvist B, Ringdèn O, Ljungman P, Wahren B, Gahrton G. Reduced risk of recurrent leukaemia in bone marrow transplant recipients after cytomegalovirus infection. Br J Haematol. 1986;63(4):671–679. - PubMed
-
- Elmaagacli AH, Steckel NK, Koldehoff M, et al. Early human cytomegalovirus replication after transplantation is associated with a decreased relapse risk: evidence for a putative virus-versus-leukemia effect in acute myeloid leukemia patients. Blood. 2011;118(5):1402–1412. - PubMed
-
- Nachbaur D, Clausen J, Kircher B. Donor cytomegalovirus seropositivity and the risk of leukemic relapse after reduced-intensity transplants. Eur J Haematol. 2006;76(5):414–419. - PubMed
-
- Remberger M, Ringdén O. Survival after bone-marrow transplantation. Lancet. 2002;359(9309):888. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA18029/CA/NCI NIH HHS/United States
- K23AI097234/AI/NIAID NIH HHS/United States
- R01 HL108307/HL/NHLBI NIH HHS/United States
- HL108307/HL/NHLBI NIH HHS/United States
- R01 AI053193/AI/NIAID NIH HHS/United States
- R01 CA136551/CA/NCI NIH HHS/United States
- P01 HL036444/HL/NHLBI NIH HHS/United States
- AI053193/AI/NIAID NIH HHS/United States
- K24 HL093294/HL/NHLBI NIH HHS/United States
- HL36444/HL/NHLBI NIH HHS/United States
- CA136551/CA/NCI NIH HHS/United States
- P01 CA018029/CA/NCI NIH HHS/United States
- P30 CA015704/CA/NCI NIH HHS/United States
- CA78902/CA/NCI NIH HHS/United States
- P30CA015704-35S6/CA/NCI NIH HHS/United States
- K23 AI097234/AI/NIAID NIH HHS/United States
- P01 CA078902/CA/NCI NIH HHS/United States
- K24HL093294/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

